FIGURE 52-1 Pedigree of patient with catecholaminergic polymorphic ventricular tachycardia (CPVT). This three-generation pedigree utilizes standard pedigree symbols (men are represented by squares; women are represented by circles). The pedigree was constructed by the genetic counselor during the proband’s first appointment in the Inherited Arrhythmia Clinic. A major “red flag” for risk assessment was present in the pedigree; the proband’s maternal uncle died suddenly at 38 years of age. Genetic testing in the proband identified a causative mutation in the RYR2 gene. Subsequently, the proband’s first-degree at-risk relatives (parents and sister) underwent cascade genetic testing, and his mother was identified to also have the RYR2 mutation.
CASE EXPLANATION
Genetic testing was able to confirm a diagnosis of CPVT in a patient with a prior, likely misdiagnosis of epilepsy. Genetic counseling is recommended for all patients with CPVT (and other inherited heart diseases) and their at-risk relatives in the Heart Rhythm Society/European Heart Rhythm Association expert consensus statement on genetic testing for channelopathies and cardiomyopathies.1 Through a multidisciplinary clinical approach that included genetic counseling, the patient and his family were able to:
• Receive an accurate diagnosis and appropriate management.
• Determine their risk status through cascade, mutation-specific genetic testing.
• Receive information regarding the inheritance pattern of CPVT and recurrence risk.
• Learn about future reproductive options.
• Receive psychosocial support and resources.
GENETIC COUNSELING AND GENETIC COUNSELORS
Genetic Counseling
Genetic counseling is the process of helping individuals and their families understand and adapt to the medical, psychological, and familial implications of a genetic condition.2 The genetic counseling process for patients with inherited heart disease includes multiple components, listed and explained in further detail below. Genetic counseling is indicated regardless of the availability of genetic testing for a specific cardiac disorder.
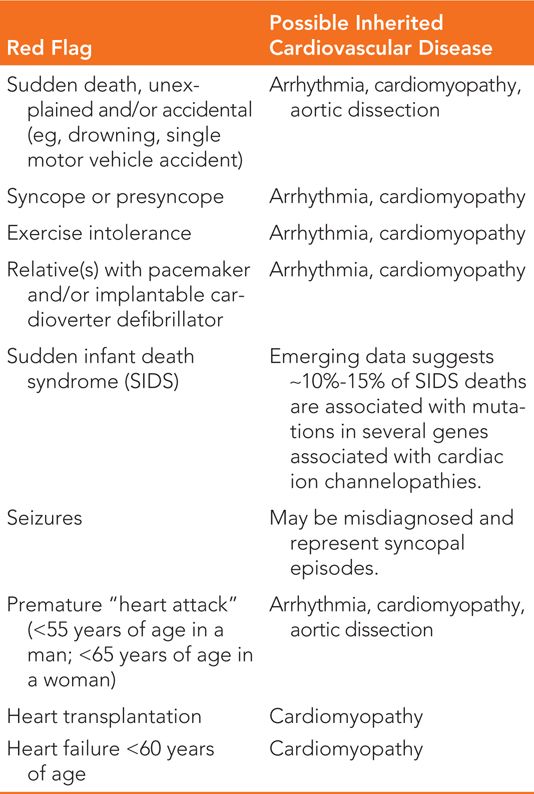
• Collection of ≥3 generation family medical history information, with special attention to “red flags” for inherited heart disease (Table 52-1).
TABLE 52-1 “Red Flags” in Pedigree That May Signify Underlying Inherited Cardiovascular Disease
 Includes confirmation with medical records, autopsy reports, and death certificates.
Includes confirmation with medical records, autopsy reports, and death certificates.
• Performance of risk assessment utilizing medical and family history information.
• Analysis and discussion of inheritance patterns and recurrence risk.
• Facilitation of genetic testing process.
 Pre- and posttest genetic counseling.
Pre- and posttest genetic counseling.
• Facilitation of family-based care.
 Cascade genetic testing (Figure 52-2).
Cascade genetic testing (Figure 52-2).
 Coordination of family clinical screening.
Coordination of family clinical screening.
• Discussion of reproductive options.
• Provision of written documentation of medical, genetic, and counseling information to referring health care providers and patients, including family letters.
• Provision of psychosocial counseling and anticipatory guidance.
• Provision of education, resources, and advocacy to patients and families.
• Discussion of available genetics research study options.
• Discussion of the availability of DNA banking, when applicable.
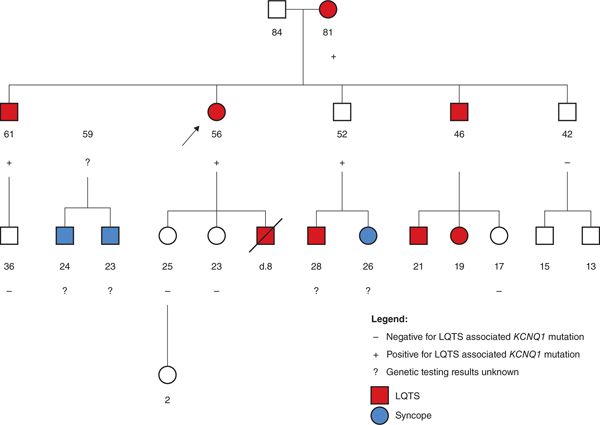
FIGURE 52-2 Implementation of cascade genetic testing in a large family with long QT syndrome (LQTS). This pedigree shows a large family with long QT syndrome (LQTS). The proband, designated by an arrow, is a 56-year-old woman who presented to the Inherited Arrhythmia Clinic due to her previous clinical diagnosis of LQTS after her 8-year-old son died suddenly while swimming. The genetic counselor met with the proband as part of her multidisciplinary consultation and this four-generation pedigree was constructed. Genetic testing had not been performed in the family to date, so the genetic counselor explained the potential benefits and limitation of LQTS genetic testing to the proband, who decided to proceed with full panel LQTS genetic testing. A pathogenic KCNQ1 mutation was identified in the proband, which allowed predictive genetic testing to be offered to her at-risk relatives. Both of her daughters tested negative for the mutation, which meant her granddaughter was not at risk for LQTS. The proband utilized a family letter provided by the genetic counselor to inform the rest of her at-risk siblings and their children about the option of cascade genetic testing. Multiple additional individuals in the family were able to determine their accurate risk level based on the genetic testing information. Several additional individuals, marked with question marks, could still benefit from this predictive genetic testing information.
Genetic Counselors
Genetic counselors are health care professionals with specialized graduate degrees and expertise in medical genetics and counseling. Many genetic counselors work as members of a health care team and provide information and support to families with inherited conditions. Cardiovascular genetic counselors are an important resource and integral health care team members for patients and families with inherited heart disease, including those families who have suffered a sudden death in a young person.3 It has been suggested that a master’s-trained, board-certified genetic counselor, preferably with specialized training in cardiovascular genetics, be part of the multidisciplinary team involved in the care of families with heritable cardiovascular diseases.4 The types of patients that should be referred to for cardiovascular genetic counseling and potentially genetic testing are listed in Table 52-2. Genetic counselors can be located by utilizing the “Find a Genetic Counselor” tool on the National Society of Genetic Counselors Web site, www.nsgc.org.
TABLE 52-2 Indications for Referral for Cardiovascular Genetic Counseling and Testing
Patients with a suspected or definite diagnosis or family history of the following: |
Inherited arrhythmias and channelopathies |
• Long Qt syndrome |
• Short Qt syndrome |
• Brugada syndrome |
• Catecholaminergic polymorphic ventricular tachycardia |
• Familial atrial fibrillation |
• Familial conduction system disease |
• Idiopathic ventricular fibrillation |
Cardiomyopathies |
• Hypertrophic cardiomyopathy |
• Idiopathic or familial dilated cardiomyopathy |
• Arrhythmogenic right ventricular cardiomyopathy |
• Left ventricular noncompaction cardiomyopathy |
• Restrictive cardiomyopathy |
Hereditary conditions affecting the aorta and other blood vessels |
• Marfan syndrome |
• Loeys-Dietz syndrome |
• Ehlers-Danlos syndrome |
• Familial thoracic aortic aneurysm and dissections |
• Premature coronary artery disease (<55 years of age in a man; <65 years of age in a woman) |
• Familial hypercholesterolemia |
• Congenital heart disease |
• Family history of sudden cardiac (or unexplained) death <50 years of age |
TAKING AN INFORMATIVE PEDIGREE: ESSENTIAL TOOL FOR CARDIOVASCULAR GENETIC MEDICINE
A careful family history for at least three generations should be collected and assessed for all patients with potential hereditary heart disease. The individual collecting the family history should actively inquire about the presence of “red flags” while constructing the pedigree since the patient themselves may not know to offer certain information important to risk assessment (eg, the presence of sudden infant death syndrome [SIDS] in a blood relative may represent the presence of a cardiac ion channel gene mutation). For a list of “red flags” and what inherited heart condition they may represent, see Table 52-1. The collection of family history is imperative in (1) aiding diagnosis, (2) identifying at-risk relatives, (3) selecting the most informative family member for genetic testing initiation, and (4) determining inheritance pattern.
While most inherited cardiac diseases follow an autosomal-dominant pattern of inheritance, there are important exceptions, and this information is necessary for the provision of accurate recurrence risks. Also complicating matters is the fact that many inherited cardiac
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree