Generations of the Plug-And-Patch Repair: Its Development and Lessons from History
Jerrold Young
Arthur I. Gilbert
Introduction
The principle of hernia repair using an open approach for placing mesh in the properitoneal retromuscular space of Bogros is based on protecting the myopectineal orifice (MPO) from behind. This has been the theory behind the development of many techniques of hernia repair including those of Nyhus, Stoppa, Kugel, and more recently, laparoscopic techniques. Furthermore, the concept of protecting an opening from inside the defect has resulted in modification of the technique of repair of other hernias, including ventral, umbilical, and particularly, incisional hernias.
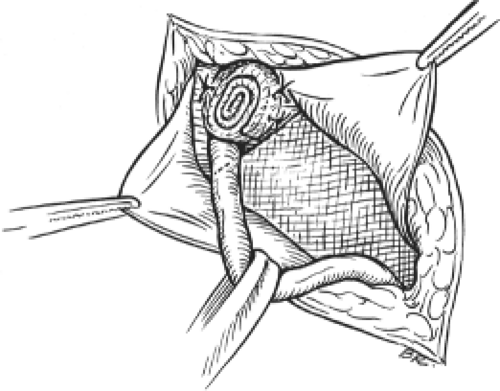
In 1985, Gilbert borrowed Lichtenstein’s idea of creating a rolled plug and used it to repair indirect inguinal hernias. The intact indirect sac was dissected and invaginated. A hand-rolled mesh plug was placed into the internal ring and two permanent sutures were used to fix it to the crura of the ring (Fig. 1). To complement this, a flat mesh patch was used to reinforce the floor of the inguinal canal over Hesselbach’s triangle. This worked well, but the plug was annoyingly palpable in some thin patients, a problem that was solved by altering the shape of the plug. Gilbert then changed to a hand-rolled plug which he placed through the internal ring into the properitoneal space, allowing it to unravel there—it became seated against the anterior abdominal wall. It literally blocked the peritoneal sac and its contents from protruding through the internal ring. Although no sutures were used, that technique proved satisfactory, providing a lasting repair for small- and medium-sized indirect hernias.
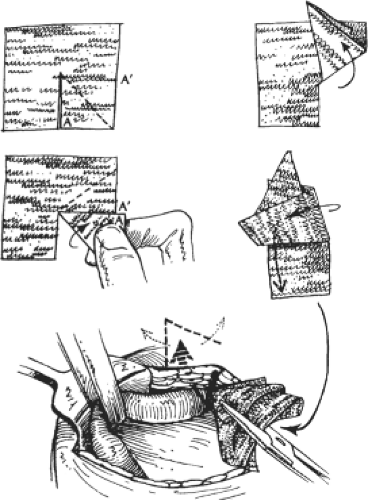
Gilbert soon recognized certain problems uniquely related to the rolled plug. These included its inability to consistently unravel enough to be anything but a wad of mesh, thus limiting its area of protection, and some patients still reported feeling it. Rarely, when a plug became infected, surgical drainage and antibiotics were insufficient to sterilize its multiple layers, compelling its removal. To avoid these problems, and to protect a wider area, the mesh shape was changed from a rolled plug to an opened patch. The flat mesh was cut, folded to the shape of a closed umbrella, and passed through the internal ring into the properitoneal space. There it was allowed to open and regain its flat contour, avoiding a palpable plug, and providing wider protection (Fig. 2). Gilbert originated the procedure of using mesh within the internal ring to repair indirect inguinal hernias, and subsequently used the internal ring as the passageway to place mesh behind the anterior abdominal wall for the repair of all types of indirect hernias.
Lessons from History—Suture Repairs
Gilbert’s approach developed from the work of earlier surgeons interested in herniology. Bassini of Padua is credited with beginning the modern era of hernia surgery. Through an anterior open approach in the groin, he divided the posterior wall of the inguinal canal, ligated the peritoneal sac, and constructed a sutured, three-layered tissue repair in 262 patients, with a failure rate of <3%. A simplification of his technique avoided opening the posterior wall, but approximated the transversus arch, or so-called conjoined tendon, to the shelving edge of the inguinal ligament with interrupted sutures. This became known as the modified (North American) Bassini repair. Lotheissen of Austria and later Anson and McVay of South Dakota, popularized an anatomic repair that approximated the transversus arch to the pectineal ligament (Cooper’s ligament), which also required opening the floor. The “McVay” repair became the preferred procedure for treatment of femoral hernias and many direct inguinal hernias. In 1946, Shouldice of Toronto
reincarnated Bassini’s original operation, and equally important, popularized the use of local anesthesia. The technically difficult Shouldice operation requires a “thinning” of the cord by dividing the lesser cord, including the genital branch of the genitofemoral nerve, and a multilayered closure of the floor with stainless steel wire. However, suturing the transversus arch to the inguinal ligament creates tension at the suture line. This feature of the Bassini operation, and other sutured repairs, is most disadvantageous. Even with relaxing incisions to reduce suture line tension, patients still experienced a high level of postoperative pain, prolonged disability, and unacceptable failure rates because of the tension.
reincarnated Bassini’s original operation, and equally important, popularized the use of local anesthesia. The technically difficult Shouldice operation requires a “thinning” of the cord by dividing the lesser cord, including the genital branch of the genitofemoral nerve, and a multilayered closure of the floor with stainless steel wire. However, suturing the transversus arch to the inguinal ligament creates tension at the suture line. This feature of the Bassini operation, and other sutured repairs, is most disadvantageous. Even with relaxing incisions to reduce suture line tension, patients still experienced a high level of postoperative pain, prolonged disability, and unacceptable failure rates because of the tension.
Tension-Free Mesh Repairs
Usher of Texas, in 1960, reported suturing a polyethylene mesh inlay patch deep to the transversalis fascia to do a “tension-eliminating” inguinal hernia repair. Newman of New Jersey developed a tension-free technique for inguinal hernia using a polypropylene mesh onlay patch that he sutured over the floor. Lichtenstein and Amid popularized Newman’s mesh technique, which has become a frequently used open, tension-free procedure for groin hernias worldwide. From 1958 to 1976, Gilbert used the modified Bassini technique with general or spinal anesthesia to repair all inguinal hernias, a technique that was estimated to have failed in 15% of primary hernias and 25% (or more) of recurrent hernias. After a visit to the Shouldice Hospital in Toronto in 1976, he began to perform the Shouldice repair under local anesthesia using 3-0 Prolene suture instead of wire. Failure rates of primary repairs dropped to <2% and of recurrent hernias to 8%. In 1984, to supplement the repair, Gilbert began placing a swatch of polypropylene mesh beneath the transversalis fascia, deep to the first suture line of the Shouldice operation. The failure rate was reduced to <0.5% for primary hernias and to 3% for recurrent hernias. Even before the open tension-free anterior repair of inguinal hernias became popular, open posterior repairs attracted surgeons’ interest through the works of Nyhus of Chicago and Condon of Milwaukee. They described musculo-aponeurotic sutured repairs, sometimes adding mesh. Stoppa and Rives of France repaired bilateral groin hernias by widely wrapping the peritoneal base with large mesh netting (giant reinforcement of the visceral sac), thereby blocking the viscera from entering any defect through either MPO. After visiting and operating with Rene Stoppa in Amiens, Gilbert was further convinced that the ideal place to position mesh is in the properitoneal space, between the force of the hernia and the defect in the abdominal wall.
Anatomical and Functional Considerations
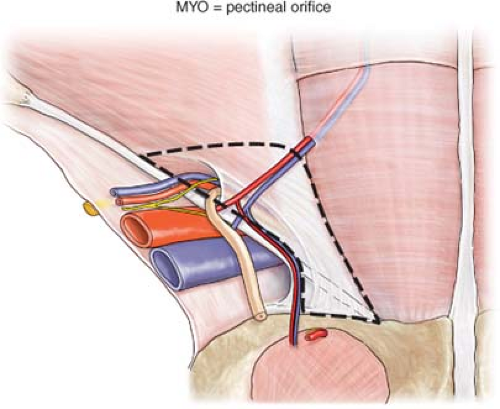
All groin hernias protrude through the MPO, a window in the lower abdominal wall surrounded by musculo-aponeurotic structures described by Fruchaud in 1956 (Fig. 3). The MPO is bounded medially by the lateral edge of the rectus muscle and its fascia, superiorly by the transversus abdominis muscle, laterally by the iliopsoas muscle, and inferiorly by the pectineal (Cooper’s) ligament. It is divided into smaller inguinal and femoral windows by the inguinal ligament anteriorly and the iliopubic tract posteriorly. The boundaries of the lateral triangle are the deep epigastric vessels, the middle third of the inguinal ligament, and a line from the junction of the upper and middle thirds of the inguinal ligament to where the deep epigastric vessels cross the lateral edge of the rectus muscle. Groin hernias occur because of poor collagen content in the MPO. The hernia that presents clinically is in that part of the MPO that initially gives way to intra-abdominal pressure. When performing any repair, one should consider the plasticity of the canal’s posterior wall and the deformation effect on vulnerable tissues that surround that defect. Appreciation of these factors is important to the long-term success of any repair in which a prosthetic device is used.
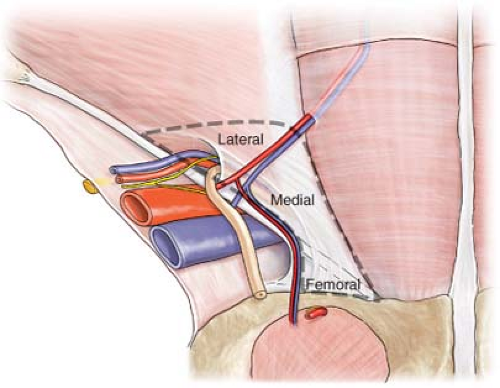
Cadaver studies indicate that the mean MPO is 7.8 cm wide and 6.5 cm high. Coverage of the MPO can be accomplished from an open or laparoscopic posterior approach, an anterior patch, or a combination of anterior and posterior coverage. Preformed or handmade mesh plugs can be placed in the internal ring of indirect hernias or in the defect of direct hernias. The problem with using a plug without a patch in any primary hernia repair is that the remainder of the canal’s unprotected posterior wall, medial and lateral to the plug, becomes subjected to an imbalance of plasticity. It is thereby rendered to have greater risk of herniation. It has been our observation that the majority of recurrences following pure tissue repairs presented in the medial triangle just above the pubic tubercle. Although half of tissue repairs that failed occurred within 5 years, other failures happened much later, some presenting decades after the original repair. On the other hand, failures following prior mesh repairs presented more commonly in the lateral triangle and became clinically evident within 2 years following the initial repair (Fig. 4). The reason for failure following mesh repair is that the mesh used initially did not cover the entire MPO, leaving the unprotected area vulnerable. On occasion, a recurrent hernia may also penetrate the floor and remain under the mesh that was placed anteriorly. It is clear that an anterior patch acts as a lid, not as a stopper.
Principles of Hernia Repair
The basic principle of every type of inguinal hernia repair is the reduction of the hernia contents and the prevention of passage of
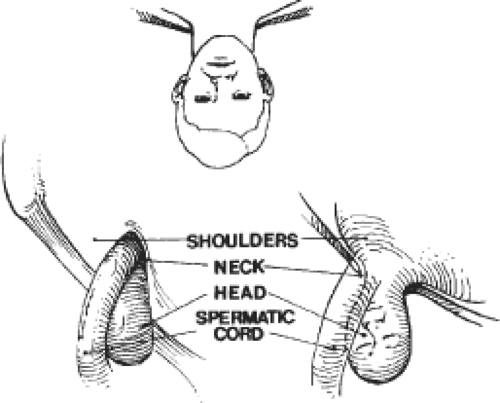
any intra-abdominal or properitoneal contents to outside the musculo-aponeurotic plane of the abdominal wall. Indirect inguinal hernia repair requires protection of the internal ring. Regardless of how the distal portion of the sac is treated, the peritoneal sac that resides within the deep inguinal ring of an indirect inguinal hernia must be carefully dissected from the cord and from the investing fibers of the transversalis fascia at the musculofascial threshold of the deep ring. A longer peritoneal sac or one densely adherent to the spermatic cord outside the internal ring should be divided, leaving its distal portion undisturbed, minimizing dissection near the spermatic cord. Further, the “shoulders” of the sac must be separated from the transversalis fascia to actualize the potential space for the prosthesis (Fig. 5). For direct hernias, the floor must be opened to allow the mesh to be placed behind the transversalis fascia.
any intra-abdominal or properitoneal contents to outside the musculo-aponeurotic plane of the abdominal wall. Indirect inguinal hernia repair requires protection of the internal ring. Regardless of how the distal portion of the sac is treated, the peritoneal sac that resides within the deep inguinal ring of an indirect inguinal hernia must be carefully dissected from the cord and from the investing fibers of the transversalis fascia at the musculofascial threshold of the deep ring. A longer peritoneal sac or one densely adherent to the spermatic cord outside the internal ring should be divided, leaving its distal portion undisturbed, minimizing dissection near the spermatic cord. Further, the “shoulders” of the sac must be separated from the transversalis fascia to actualize the potential space for the prosthesis (Fig. 5). For direct hernias, the floor must be opened to allow the mesh to be placed behind the transversalis fascia.
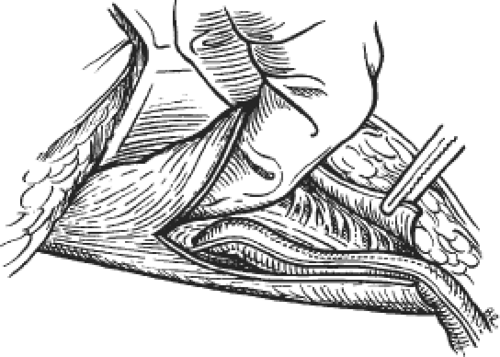
In Gilbert’s original technique, and its two later generations, the mesh was interposed between the peritoneum and the transversalis fascia (the properitoneal space of Bogros) (Fig. 6). The principle of those repairs is that when the patient’s intra-abdominal force is applied against the mesh, it will hold the patch in place to fortify the area covered. Each generation of technique incorporates the same three features: (a) the deep (internal) inguinal ring is an available and convenient passageway to be used to get to the properitoneal retromuscular space, (b) polypropylene mesh is an excellent permanent barrier to protect the deep inguinal ring, and (c) the body’s intra-abdominal pressure is sufficient to permanently fix the mesh in its properitoneal position (Pascal’s principle). Until April 1998, Gilbert used a tension-free polypropylene umbrella patch technique to repair all indirect inguinal hernias, and the Shouldice repair with a reinforcing underlay mesh patch (not tension-free) to repair all direct hernias. In doing so, the plug covered the primary defect from behind and the patch functions as an overlay on the full MPO.
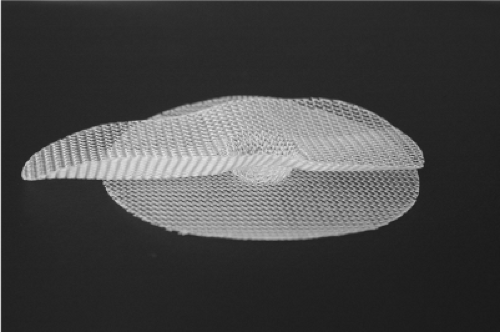
Primarily because of the complexity of the anatomy, over the years, there have been many different techniques described for inguinal hernia repair. The ideal hernia repair would be performed as an out-patient procedure under local anesthesia, with a short operative time, minimal post-op and long-term discomfort and disability, at low cost, with low risk for other side effects and complications, and have very few recurrences. The technique should have a short learning curve, with excellent reproducible results when performed by all general surgeons as well as experts. There is no single repair that has all of these desired outcomes—hence the continued investigation and analysis of new concepts and techniques. In 1997, Gilbert, with a personal experience of over 20,000 hernia repairs, designed the Prolene hernia system (PHS) (Fig. 7)—a polypropylene bilayer connected mesh device used to
repair all types of direct and indirect inguinal hernias through an open anterior approach. The system has three components: a flat round underlay, an elongated overlay (oval shaped with flat edges), and a 1.5-cm round connector that joins these in the center. Three sizes—medium, large, and extra large—are available. The PHS is symmetrical in the longitudinal axis, and can be used on either the right or left side.
repair all types of direct and indirect inguinal hernias through an open anterior approach. The system has three components: a flat round underlay, an elongated overlay (oval shaped with flat edges), and a 1.5-cm round connector that joins these in the center. Three sizes—medium, large, and extra large—are available. The PHS is symmetrical in the longitudinal axis, and can be used on either the right or left side.
Quality-Of-Life Issues
As a result of the use of mesh and improved surgical technique, recurrence rates after hernia surgery have been reduced. Increased attention has been directed to quality-of-life issues, particularly the problem of chronic pain—a common consequence occurring in up to 10% of patients after hernia surgery. These symptoms of somatic, visceral, and neuropathic pain, as well as testicular pain, dysejaculation, and claims of sterility have been enough to warrant discussion at surgical meetings and in the literature. Post-op pain problems are in part related to the scarring, which occurs in both non-mesh and mesh repairs. The incidence of post-op chronic pain is greater in patients with suture repairs than in those with tension-free mesh repairs. Placement of mesh results in immediate strength of the repair. In addition, the mesh induces an inflammatory reaction and scarring, making the repair stronger as the scar creates a plate of tissue. This inflammatory reaction may affect structures, which are in direct apposition to the mesh, a situation that is present in all types of hernia repairs. Lateral to the internal ring, the mesh is placed on top of the internal oblique, and unavoidably comes into contact with the ilioinguinal and iliohypogastric nerves, which may result in inflammation and scarring involving these structures. In routine hernia repairs, nerves, muscle, the spermatic cord, and all structures in the inguinal canal may come in contact with the mesh. This is true in open anterior or properitoneal repairs, as well as in laparoscopic repairs.
There have been suggestions on how some of these post-op consequences may be reduced by modifying the surgical technique. These include avoiding nerve trauma by blunt dissection, traction, and electrocautery; limiting dissection close to the spermatic cord to reduce scarring that may result in cord dysfunction, obstruction, and possible injury to the nerves and vessels that are present in the adventitia of the vas; avoiding placement of mesh in direct opposition to the vas when possible; using absorbable sutures with air knots and placing sutures in the internal oblique away from visible nerves; dividing a long indirect sac near the internal ring and avoid dissecting near the spermatic cord distally; and avoiding placement of sutures into the periosteum of the pubic tubercle. New products such as lightweight mesh, omega-3 fatty acid-coated mesh (C-Cur Lite®), and tissue glue instead of sutures are being investigated in an attempt to reduce inflammatory reaction and scarring, and possibly improve results. Further evidence-based studies are needed to document the efficacy of these concepts and products. However, preliminary reports indicate that many of these suggestions have sufficient basis to begin implementing them. As a result of this information, we have modified our surgical technique to attempt to limit dissection in the area of the nerves and the spermatic cord to attempt to reduce the incidence of these problems. In time, we will learn if some of the experimental data will hold up in the clinical setting. In addition, “watchful waiting”—conservative management of asymptomatic hernias—is an acceptable course of management in the appropriate patient (Table 1).
Table 1 Patient Demographics by Time Period | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Current Technique
Preoperative Planning
When indicated, medical evaluation and clearance is requested. Groin and testicular ultrasound examination is often done in patients who have recurrent hernias or patients with testicular complaints, to document the location of the hernia, to check for multiple hernias, and to document testicular anatomy and blood flow. Appropriate informed consent is obtained—we have patients sign a “hernia-specific” informed consent, which is sent to the surgery center to become a part of the record. Although we have rarely seen bleeding problems related to Aspirin® and non-steroidal anti-inflammatory drugs, these drugs as well as Plavix® are stopped 7 to 10 days before surgery. If the patient takes Coumadin®, it is stopped 5 days before surgery. Depending on the indication for the anticoagulation, some patients require a short-acting subcutaneous anticoagulant (Lovenox® or heparin) for the immediate pre-op and post-op period. Our current approach is to discuss this with the medical consultant familiar with the patient, who then will order these medicines. Clotting studies are done on the day of the operation. All other medications are continued up until midnight before surgery. The patient is advised to shower the morning of surgery using a parachlorometaxylenol-impregnated sponge to bathe the abdominal and groin areas, but not to shave the surgical site. In the pre-op suite, the patient is identified and the surgical site is confirmed and marked prior to administration of any sedative. Any hair at the operative site is clipped just prior to the surgery. A single dose of 1 g of cefazolin (or 600 mg of clindamycin for penicillin- or cephalosporin-allergic patients) is administered within 30 minutes of incision time (Table 2).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree