Fig. 3.1
Chemical structure of triptans
The indole structure of triptans is identical to the neurotransmitter 5-HT. Triptan structure contains side chain on the indole ring. The main structural difference of triptans is the presence of the sulfonamide with a different side chain attached to it at position 5 and the presence of a nitrogen-alkyl chain at position 3. Rizatriptan, zolmitriptan, and frovatriptan have, respectively, a 2-oxazolidone and an amide instead of a sulfonamide, a triazole. In the chemical structure of eletriptan, the nitrogen-alkyl chain connected to the indole ring is replaced with a dimethyl-pyrrolidine, and in naratriptan with a 1-methyl-piperidine ring. Triptans are characterized by three main mechanisms of action, all contributing to their anti-migraine effects. These effects include: (i) the peripheral inhibition of the vasoactive peptides release from trigeminal nociceptive afferents; (ii) the cranial vasoconstriction; and (iii) the inhibition of the second-order neurons transmission through the trigeminocervical complex. There is also evidence that they may be acting in other brainstem nuclei and the thalamus [4–6].
Nowadays, triptans are considered the first-line option in the acute treatment of moderate–severe migraine attacks. Cardiovascular and cerebrovascular diseases represent the main contraindication for the prescription of triptans, although the clinical significance of triptan vasoconstriction is unclear and still being debated.
3.3.2 NSAIDs
Nonsteroidal anti-inflammatory drugs (NSAIDs) are a class of molecules that have analgesic, antipyretic and, in higher doses, anti-inflammatory effects. Chemically, most NSAIDs are organic acids with low pKa values. This feature favors their accumulation at inflammation sites, considering that these areas often exhibit low pH values. Moreover, low pKa values are also related to short half-lives. Most NSAIDs can be classified into two groups based on their chemical structure: carboxylic acid and enolic acid derivatives (Table 3.1). Carboxylic acid subgroups include the salicylates, arylpropionic acids, anthranilic acids, and phenylacetic acids. The main subgroups of enolic acids are pyrazolones and oxicams. NSAIDs can be classified on chemical structure or mechanism of action. Newer molecules are frequently classified by mechanism of action.
Table 3.1
Classification of NSAIDs
Carboxylic acids | Arylpropionic acids | Flurbiprofen, ketoprofen, oxaproxin, ibuprofen, naproxen, fenoprofen | |
Salicylic acids | Aspirin, difunisal, trisalicylate salsalate, sodium salicylate, olsalazine, sulfasalazine | ||
Anthranilic acids | Mefenamic acid, meclofenamic acid | ||
Acetic acids | Indole and indene acids | Etodolac, indomethacin, sulindac, tolmetin, ketorolac | |
Phenylacetic | Diclofenac | ||
Enolic acids | Pyrazolones | Phenylbutazone | |
Oxicams | Piroxicam, meloxicam | ||
Nonacidic compounds | Nabumetone | ||
COX-2 selective inhibitors (Coxibs) | Colecoxib, rofecoxib, meloxicam, nimesulide, paracoxib | Etodolac, lumiracoxib, valdecoxib, deracoxib, etoricoxib | |
The term NSAID is used for compounds that inhibit the metabolism of arachidonic acid (AA). NSAIDs inhibit the synthesis of prostaglandins and thromboxanes, modifying the activity of both cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2), which catalyzes the formation of prostaglandins responsible for pain and inflammation. Experts believe that the inhibition of COX-2 leads to the anti-inflammatory, analgesic, and antipyretic effects and that those NSAIDs, inhibiting also COX-1 and particularly aspirin, may cause gastrointestinal bleeding and ulcers [7]. Most of selective COX-2 inhibitors are diarylheterocycles. NSAIDs that inhibit prostaglandin E2 synthesis are effective in treating acute migraine attacks. Ibuprofen, paracetamol, acetylsalicylic acid, lysine acetylsalicylate, naproxen sodium, diclofenac sodium, and potassium and ketorolac have the highest efficacy in migraine treatment, whereas the evidence of efficacy for other NSAIDs is more limited.
Ibuprofen is a nonselective inhibitor of cyclooxygenase, an enzyme involved in prostaglandin synthesis via the arachidonic acid pathway, but its exact mechanism of action is yet unknown. It is administered as a racemic mixture. The R-enantiomer goes through interconversion to the S-enantiomer in vivo. It is believed that the S-enantiomer is the more pharmacologically active enantiomer. Its pharmacological effects might be due to inhibition of cyclooxygenase-2 (COX-2), which decreases the synthesis of prostaglandins involved in mediating inflammation, pain, fever, and swelling. Side effects of ibuprofen, such as GI ulceration, are due to its inhibition of COX-1.
Paracetamol is an analgesic antipyretic derivative of acetanilide and it has weak anti-inflammatory properties. It is the drug of choice for adult patients when salicylates or other NSAIDs are contraindicated. The lack of significant anti-inflammatory activity of paracetamol implies a mode of action distinct from that of NSAIDs; yet, despite years of use and research, the mechanisms of action of paracetamol are not fully understood even if it is now being considered as a selective COX-2 inhibitor.
3.3.3 Ergot Derivatives
Ergotamine and dihydroergotamine activate 5-HT1B receptors located on intracranial blood vessels, leading to vasoconstriction correlated with the relief of migraine headache; they also act on the inhibition of pro-inflammatory neuropeptide release by activating 5-HT1D receptors on sensory nerve endings of the trigeminal system [8]. Ergot derivatives have a potential risk of abuse; therefore, their use should be restricted to low-frequency severe attacks unresponsive to other treatments. The ergot alkaloids were found in ergot fungi (genus Claviceps) and, in their chemical structure, they present the basic compound ergoline, the structural skeleton of several alkaloids (Fig. 3.2a).
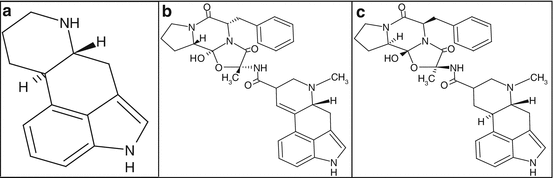

Fig. 3.2
Chemical structure of ergot alkaloids. (a) Ergoline; (b) ergotamine; (c) dihydroergotamine
In particular, ergotamine is an ergopeptine and dihydroergotamine is a 9,10 alpha-dihydro derivative of ergotamine (Fig 3.2b, c). They have a complex mechanism of action due to the interaction with several receptors. In fact, both ergotamine and dihydroergotamine demonstrate affinities for 5-hydroxytryptamine, dopamine, and noradrenaline receptors since they have a structure similar to these neurotransmitters.
3.3.4 Antiemetics
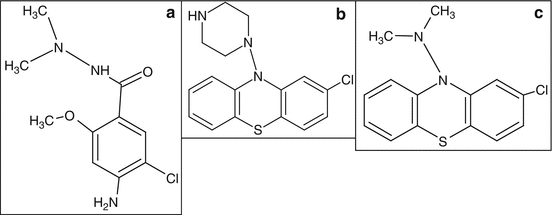
Antiemetics, including metoclopramide, prochlorperazine, and chlorpromazine, are effective treatment options for migraine independently from their ability to control nausea and vomiting. They are considered primary options for treating acute migraine in the emergency department setting. They are to be considered adjuvants in the treatment of migraine attacks, especially when nausea or vomiting is prominent: the efficacy of analgesics is reduced in many migraineurs because of impaired gastrointestinal motility, which is associated with nausea, and the nonabsorption of the drugs due to vomiting. For instance, NSAIDs are often combined with an antiemetic for migraine pain treatment to reduce associated nausea and vomiting [9]. It is also true that the dopamine antagonist properties of metoclopramide might make it effective as single treatment for acute migraine. Metoclopramide belongs to the salicylamides, carboxamide derivatives of salicylic acid. Other dopamine antagonists such as prochlorperazine and chlorpromazine have also shown effectiveness in migraine (Fig. 3.3). These polycyclic aromatic compounds belong to the chemical class of phenothiazine, which is a linear tricyclic system that consists of two benzene rings united by a para-thiazine ring.
 < div class='tao-gold-member'>
< div class='tao-gold-member'>





Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


