and Peter Hamilton2
(1)
Department of Medicine, University of Alberta, Edmonton, AB, Canada
(2)
Division of General Internal Medicine, Department of Medicine, University of Alberta, Edmonton, AB, Canada
Approach to Diagnostic Tests and Clinical Trials
Diagnostic Tests
2 × 2 TABLE
Disease present | Disease absent | Total | |
|---|---|---|---|
Test positive | a (true positive) | b (false positive) | a + b |
Test negative | c (false negative) | d (true negative) | c + d |
Total | a + c | b + d | a + b + c + d |
SENSITIVITY ★SNOUT★
=a/(a + c)
=out of 100 patients with disease, how many have a positive test result? Independent of prevalence and helps to rule out disease
SPECIFICITY ★SPIN★
=d/(b + d)
=out of 100 people without disease, how many have a negative test result? Independent of prevalence and helps to rule in disease
POSITIVE PREDICTIVE VALUE (PPV)
=a/(a + b)
=out of 100 patients with a positive test result, how many actually have disease? Dependent on prevalence of disease
NEGATIVE PREDICTIVE VALUE (NPV)
=d/(c + d)
=out of 100 patients with a negative test result, how many do not have disease? Dependent on prevalence of disease
LIKELIHOOD RATIOS (LR)
—indicates how much a given diagnostic test result will change the pretest probability of the disorder under investigation:
LR 1.0 means pre-test probability = post-test probability
LR >1.0 increases the probability the disorder is present. A test with LR >10 is particularly useful
LR <1.0 decreases the probability the disorder is present. A test with LR <0.1 is particularly useful
POSITIVE LIKELIHOOD RATIO (LR+)
=(positive test in disease)/(positive test in no disease)
=sensitivity/(1 – specificity)
NEGATIVE LIKELIHOOD RATIO (LR–)
=(negative test in disease)/(negative test in no disease)
=(1 – sensitivity)/specificity
ACCURACY
=(a + d)/(a + b + c + d)
=how often is test correct in predicting true positive and true negative
TO CALCULATE THE POST-TEST PROBABILITY OF A DIAGNOSIS AFTER A TEST
pre – test probability
=probability of disease just prior to performing test of interest
=disease prevalence (if no other diagnostic test previously performed) or post-test probability (after other initial investigations)
pre – test odds = pre-test probability/(1 – pre-test probability)
post – test odds = pre-test odds × likelihood ratio
post test probability = (post-test odds)/(1 + posttest odds)
FAGAN NOMOGRAM
—easily converts from pre-test probability to post-test probability using LR (alleviating tedious calculations above)
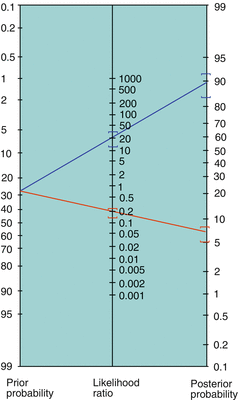
Reprinted with permission from eMedicine.com, 2009. Available at: http://emedicine.medscape.com/article/773832-overview
Therapeutic Interventions
2 × 2 TABLE
Outcomepositive | Outcomenegative | Total | |
|---|---|---|---|
Exposurepositive | A | B | a + b |
Exposurenegative | C | D | c + d |
Total | a + c | b + d | a + b + c + d |
ODDS RATIO (OR)
– case control study
= ad/bc. Odds ratio approximates RR if the disease is relatively rare
RELATIVE RISK (RR)
– cohort study
= [a/(a + b)]/[c/(c + d)]
= probability of disease in exposed patients/probability of disease in patients without exposure
RELATIVE RISK REDUCTION (RRR)
= [a/(a + b) – c/(c + d)]/[c/(c + d)]
ABSOLUTE RISK REDUCTION (ARR)
= a/(a + b) – c/(c + d)
NUMBER NEEDED TO TREAT (NNT)
=1/ARR = number of patients you would need to treat for one patient to benefit from the treatment of interest
Smoking Cessation
NEJM 2002 346:7; NEJM 2008 359:19
Complications and Smoking-Associated Disorders
CANCER
—lung, head and neck (larynx, pharynx, oral cavity), esophagus, pancreas, bladder, kidney, stomach, cervix, AML
CARDIOVASCULAR DISEASES
—CAD, CVD, PVD, Buerger’s disease
RESPIRATORY DISEASES
—COPD, pneumonia
METABOLIC
—diabetes mellitus, infertility, premature menopause, osteoporosis
COAGULOPATHY
MORTALITY
—all-cause mortality ~3-fold higher (death most commonly from neoplastic, vascular or respiratory causes)
Pathophysiology of Smoking
NICOTINE ADDICTION
—related to the combination of the following: (1) the pleasurable effects of nicotine (e.g. relief of anxiety and arousal); (2) the pleasurable effects of associated environmental triggers (e.g. coffee and meals); and (3) the unpleasureable effects of nicotine withdrawal (e.g. dysphoria, anxiety, irritability, insomnia, decreased concentration, increased appetite and over the long-term increased weight)
LUNG CANCER
—cigarette smoke contains numerous carcinogenic substances. In particular, N-nitrosamines and polycyclic aromatic hydrocarbons are metabolized to nitrosamine ketone and N′-nitroso-nornicotine by the cytochrome P450 system, which form DNA adducts, leading to mutations and eventually cancer. The duration of cigarette exposure is a greater risk factor than the number of cigarettes smoked per day. Cigarette smoking is a greater risk factor than pipe and cigar smoking. Smokers have a 10–30× increased risk of developing lung cancer. The risk the lung cancer returns close to baseline (i.e. 80–90% reduction) after 10–15 years of smoking cessation. Second-hand smokers have up to 2× increased risk of lung cancer
LIFE EXPECTANCY
—on average, 13.2 and 14.5 years shorter for male and female smokers compared to non-smokers, respectively. Smoking cessation between 45 and 54 years of age reduces risk of death associated with continued smoking by two-thirds.
Management of Smoking Cessation
COUNSELING
—identify smoking cues and use cognitive and behavioral methods to break the link. Remove cues (remove ash trays, avoid settings where smoking occurs, suggest other smokers in the household to quit at the same time, or other substances). Coping (inform family/friends/co-workers about quitting and seek support, plan strategies such as gum, stress management)
DRUG THERAPY
—nicotine replacement (nicotine gum, nicotine transdermal 21 mg daily × 6 weeks, then 14 mg daily × 2 weeks, then 7 mg daily × 2 weeks). Bupropion SR (150 mg PO daily × 3 days, then BID × 7–12 weeks, stop smoking after 6–7 days of treatment). Nicotinic acetylcholine receptor partial agonist (varenicline 0.5 mg PO daily for d1–3, then 0.5 mg PO BID d4–7, then 1 mg PO BID for weeks 2–12). E-cigarettes/personal vaporizers may assist with abstinence but are not currently FDA-approved for smoking cessation
Treatment Issues
APPROACH TO COUNSELING
1.
screening—identification of smokers at every visit and explore willingness to quit
2.
explore patient ’ s own reasons to quit—current health, social (e.g. children), or economic issues. Explain comorbidities associated with smoking. “As your doctor, I need you to know that quitting smoking is the most important thing you can do to protect your health”
3.
if patient ready to quit within 30 days—offer counseling (quit date, what worked, what did not, express confidence, strategies) and aid (nicotine replacement, bupropion)
4.
if patient wants to quit but not now—explore barriers to smoking cessation (nicotine dependence, fear of failure, lack of social support, lack of self-confidence, concern about weight gain, depression, substance abuse). Explore reasons to quit (health, social, financial). Set quit date. Follow-up
5.
if patient not ready to quit—avoid argument. Explore smoker’s view of pros/cons of smoking and cessation and correct misperceptions. Discuss risks of passive smoking for family and friends. Advise no-smoking policy at home. Offer to help the smoker when ready to quit
OBSTACLES TO CESSATION
weight gain after cessation—2.3–4.5 kg [5–10 lb]
physiological—withdrawal symptoms (see pathophysiology) usually begin few hours after the last cigarette, peak 2–3 days later, and wane over several weeks
psychological—smoking is a learned behavior/ritual. High risk of relapse (40% at 5 years); requires clinical follow-up
SIDE EFFECTS OF SMOKING CESSATION METHODS
nicotine gum—mouth irritation, sore jaw, dyspepsia, hiccups, and damage to dental work
nicotine patch—skin irritation and insomnia. Contraindications include unstable angina or MI <2 weeks, pheochromocytoma (increases catecholamine release) and pregnancy
bupropion SR—discontinuation rate of around 10%. Insomnia, dry mouth, agitation, increased risk of seizure <0.1%. May increase the risk of suicide. Enters breast milk
varenicline—dose-related nausea and vomiting. Insomnia, abnormal dreams, headaches, constipation, diarrhea, flatulence, and dyspepsia. Contraindicated in pregnancy. Use with CAUTION in mood disorders as may increase suicidal ideation
Prognostic Issues
CESSATION RATE
without help— <10%
combine drug therapy with counseling—40–60% at the end of drug treatment, 25–30% at 1 year. The use of drug therapy (either nicotine replacement or bupropion) increases success rate by 2–3× compared to placebo
Related Topics
Coronary Artery Disease (p. 28)
Esophageal cancer (p. 215)
Lung cancer (p. 203)
Multisystem Disorders
Selected Multisystem Disorders
INFECTIONS
bacterial—endocarditis, TB, Whipple’s
viral—HIV, HBV, HCV, EBV, CMV
fungal—histoplasmosis, aspergillosis
parasitic—schistosomiasis
MALIGNANCY
solid—metastatic, paraneoplastic
lymphoproliferative—leukemia, lymphoma
INFLAMMATORY
—vasculitis, rheumatoid arthritis, scleroderma, SLE, IBD
IATROGENIC
—drugs
INFILTRATIVE
—cryoglobulinemia, hemochromatosis, amyloidosis, sarcoidosis, porphyria
ENDOCRINE
—diabetes, hyperthyroidism
Hemochromatosis
INHERITANCE
—autosomal recessive. Among the North American population of European descent, approximately 10% are heterozygous and 0.4% are homozygous for hemochromatosis
PATHOPHYSIOLOGY
—mutation of HFE gene (C282Y and H63D most common mutations); normally forms a complex with transferrin receptor to decrease its affinity for transferrin → mutation causes ↑ absorption of Fe → iron deposition in organs
CLINICAL FEATURES
—skin (bronze), joints (destructive arthritis, classically 2nd and 3rd MCP), heart (arrhythmia, heart failure), pancreas (“bronze” diabetes), thyroid (hypothyroidism), liver (↑ LFT, cirrhosis, hepatocellular carcinoma 200× ↑ risk, cholangiocarcinoma rare), gonads (hypogonadism, impotence), pituitary (hypopituitarism), infections (Listeria, Yersinia, Vibrio)
DIAGNOSIS
—transferrin % saturation (=serum iron/TIBC × 100%, ↑, most useful for screening), Fe (↑), TIBC, ferritin (↑), liver biopsy (hepatic iron index), HFE genotype testing
TREATMENTS
—alcohol cessation, phlebotomy (remove 1–2 U weekly until ferritin <50 ng/mL). Chelation only if phlebotomy contraindicated (e.g. moderate/severe anemia) or not tolerated (e.g. hypotension)
Lancet 2007 370:9602
Sarcoidosis
PATHOPHYSIOLOGY
—cause unknown but may involve antigen exposure → activation of T-cell immunity → non-caseating granuloma formation
CLINICAL FEATURES
—constitutional (fatigue, weight loss, fever), pulmonary (staged according to CXR: stage I = bilateral hilar adenopathy, stage II = hilar adenopathy with parenchymal reticular opacities, upper > lower, stage III = parenchymal reticular opacities without hilar adenopathy, stage IV = advanced fibrosis with evidence of volume loss, honey-combing, hilar retraction, bullae, cysts, and emphysema. Stages not necessarily chronological), cardiac (arrhythmia especially conduction blocks, HF), GI tract (hepatomegaly, rarely ulcers, obstruction), renal (interstitial nephritis, nephrocalcinosis), neurologic (cranial nerve palsies especially CN VII, pituitary dysfunction, peripheral neuropathy, neuromuscular, transverse myelitis), ocular (uveitis), endocrine (hypercalcemia, hypopituitarism), lymphatics (lymphadenopathy, hypersplenism), joints/bone (symmetrical acute polyarthritis of ankles, chronic arthritis of large or small joints of hands and feet, bone pain with periosteal resorption), and skin (erythema nodosum, lupus pernio). Lofgren’s syndrome is an acute presentation characterized by bilateral hilar lymphadenopathy, erythema nodosum, arthritis, fever, ±uveitis (50%). It is associated with a good prognosis with >80% remission in 2 years
INVESTIGATIONS
—blood tests (CBCD, lytes, urea, Cr, Ca, PO4, AST, ALT, ALP, bilirubin, serum ACE level), urine tests (urinalysis), imaging (CXR, high resolution CT chest), special (TB skin test, ECG, PFT, LP if neurological symptoms, BAL, biopsy, ophthalmology referral). Diagnosis is made by clinical findings plus biopsy (except if Lofgren’s syndrome)
PROGNOSIS
—poor prognostic factors include age at onset >40, black race, progressive pulmonary sarcoidosis, neurological or cardiac involvement, chronic uveitis, lupus pernio, chronic hypercalcemia, and nephrocalcinosis
TREATMENTS
lung involvement—observation only if asymptomatic, minimal parenchymal changes, Lofgren’s syndrome, or stage I lung disease as high chance of spontaneous remission. Inhaled steroids for mild disease (budesonide 800–1600 mcg BID × 4–8 weeks) and systemic steroid (prednisone 1 mg/kg PO daily × 4–6 weeks) for moderate/severe disease
skin and eye involvement—topical steroid
joint involvement—NSAIDs/colchicine
cardiac or neurologic involvement or any other progressive disease—prednisone 0.5–1 mg/kg PO daily, methotrexate, azathioprine, cyclophosphamide and infliximab
Lancet 2014 383:9923
Amyloidosis
PATHOPHYSIOLOGY
—soluble amyloid precursor protein (AL = Ig light chain variable region in plasma cell dyscrasias, AA = serum amyloid A in chronic inflammatory conditions, ATTR = derived from mutant transthyretin protein, Aβ = Aβ protein precursor in Alzheimer’s, β2-microglobulin in advanced chronic kidney disease/chronic hemodialysis) → insoluble fibrils in anti-parallel β-pleated sheet configuration → deposition in different organs
CLINICAL FEATURES
—constitutional (fatigue, weight loss), renal (nephrotic proteinuria, distal RTA, nephrogenic diabetes insipidus), cardiac (HF, cardiomyopathy, arrhythmia, heart block, MI), neurologic (mixed sensory/motor peripheral neuropathy, autonomic neuropathy, bowel/bladder dysfunction, intracranial bleeding), pulmonary (pleural effusion, parenchymal nodules, tracheobronchial infiltration), GI tract (GI bleed, malabsorption, pseudoobstruction/dysmotility), hepatic (hepatomegaly), hematologic (bruising, factor X deficiency, binding of Ca-dependent factors to amyloid), endocrine (adrenal insufficiency, hypothyroidism), soft tissues (muscle pseudo-hypertrophy, shoulder pad sign, nail dystrophy, alopecia, macroglossia which is specific to AL, occurring in 20%)
DIAGNOSIS
—serum and urine protein electrophoresis, biopsy of involved organ, subcutaneous fat, rectal tissue, and bone marrow biopsy. Immunofixation electrophoresis (AL), immunohistochemical staining for specific amyloid protein (AA). Amyloid stains red with Congo red dye and shows “apple-green” birefringence under polarized light
PROGNOSIS
—median survival is 1–2 years for AL, but only 6 months if cardiac involvement. Up to 15 years in ATTR. Prognosis is dependent on underlying disease in AA
TREATMENTS
—supportive (dialysis if renal failure). Treatment of underlying infectious/inflammatory disorder (AA) and plasma cell dyscrasia (AL)
NOTE
—amyloidosis usually involves λ light chain, whereas light chain deposition disease involves κ light chain
NEJM 1997 337:13
Cryoglobulinemia
PATHOPHYSIOLOGY
—chronic immune stimulation or lymphoproliferation resulting in production of immunoglobulin (mono- or polyclonal), i.e. cryoglobulin (type I = monoclonal IgG/IgM/IgA/free light chains, produced by Waldenstrom’s macroglobulinemia/myeloma/lymphoproliferative disorder; type II = monoclonal IgM/IgA against polyclonal Ig, may be essential or due to persistent viral infections [HCV/HIV]; type III = polyclonal Ig against polyclonal Ig, may be essential or due to connective tissue diseases) → cryoglobulin precipitates with complexes at temperature <37 °C [<98.6 °F] → hyperviscosity and deposition in organs/vessels → systemic inflammation/vasculitis
CLINICAL FEATURES OF TYPE I
—skin (livedo reticularis, purpura), hyperviscosity/thrombosis (Raynaud’s phenomenon, digital ischemia)
CLINICAL FEATURES OF TYPE II/III
—constitutional (fatigue, weight loss, arthralgia, myalgia), neurologic (peripheral neuropathy), renal (proteinuria, hematuria, MPGN, RPGN), pulmonary (small airway disease), rheumatologic (Sjogren’s, Raynaud’s), splenomegaly, lymphadenopathy
DIAGNOSIS
—laboratory (↑ cryoglobulin level >800 μg/L or cryocrit >1% over 3–6 months, hypocomplementemia, ↑ ESR/CRP), clinical (vasculitis, thrombosis), pathological (biopsy of affected organ), secondary causes (serum protein electrophoresis, ANA, RF, HCV, HBV, HIV serology)
PROGNOSIS
—10-year survival 50%. Death usually due to infection or cardiovascular disease. At risk for end-stage renal disease, secondary non-Hodgkin lymphoma



