General Concepts in Pathology
Define pathology:
The study of suffering (from the Greek pathos), or the study of functional changes in cells, tissues, and organs that underlie disease
Define homeostasis:
State of internal equilibrium at which normal physiologic demands of a cell are met; pathophysiology results when stimuli (ie, cell injury) sufficiently disrupt homeostasis.
What are some mechanisms of cell injury?
Altered physiological stimuli; reduced oxygen supply; microbial infection; metabolic alteration; cumulative aging
What are the ways that cells adapt to stress?
Hyperplasia; hypertrophy; atrophy; metaplasia
Define hyperplasia:
An increase in number of cells as an adaptive response to stress, usually resulting in increased volume of an organ or tissue. Cells must be capable of mitotic division (eg, prostate).
Define hypertrophy:
An increase in cell size due to synthesis of cellular structural components as an adaptive response to stress, usually resulting in increased size of an organ or tissue. Does not require mitotic division (eg, myocardium).
Define atrophy:
Reduction of cell size due to loss of structural components of the cell. An attempt by the cell to reduce demand to match reduced supply. The entire tissue/organ diminishes in size when enough cells are involved.
Give examples of physiologic atrophy:
Loss of certain embryologic structures (eg, digit web-space, umbilicus); uterus returning to nongravid state after parturition
What are some causes of pathologic atrophy?
Hypoxia, loss of innervation, disuse, and aging
What is the process of reversible change that occurs when one adult cell type is replaced by another adult cell type?
Metaplasia
What are the hallmarks of reversible cell injury?
Reduced oxidative phosphorylation, adenosine triphosphate (ATP) depletion, cellular swelling, ion efflux, and water influx
When does irreversible cell injury occur?
This is highly variable and largely dependent on the cell/tissue type. Continued insult can eventually lead to irreversible cell injury but the threshold at which irreversible cell injury occurs is different in different tissue types. Irreversibly injured cells invariably undergo cell death.
Define karyolysis:
Dissolution of the nucleus (karyo- = nucleus, -lysis = to break apart)
Define karyorrhexis:
Nuclear fragmentation (karyo- = nucleus, -rrhexis = rupture)
Define pyknosis:
Nuclear shrinkage and condensation
What are the two main types of cell death?
- Apoptosis
- Necrosis
Define apoptosis:
A process of cell death by which a cell activates enzymes (“caspases”) that degrade the cell’s own DNA and proteins (ie, “programmed cell death”) while maintaining an intact plasma membrane. The entire cell is then phagocytized.
Define necrosis:
A process of cell death by lysosomal enzymatic digestion and loss of plasma membrane integrity
What are the key differences between apoptosis and necrosis?
Apoptosis may be physiologic or pathologic whereas necrosis is always pathologic; due to loss of plasma membrane integrity, necrosis often elicits inflammation in adjacent tissue; in necrosis, lysosomal enzymes may come from the dead cells (ie, autolysis) or from leukocytes.
Describe the steps of apoptosis:
Cell shrinkage, chromatin condensation and fragmentation, formation of apoptotic bodies, phagocytosis by macrophages.
What are the three pathways which may initiate apoptosis?
- Intrinsic (mitochondrial) pathway
- Extrinsic (death receptor-initiated) pathway
- Cytotoxic T-lymphocyte mediated pathway; all three converge on executioner caspases to initiate the execution phase of apoptosis
What are the examples of triggers of apoptosis via the intrinsic pathway?
Lack of hormonal or growth factor stimulation, DNA damage leading to p53 activation
What are the examples of triggers of apoptosis via the extrinsic pathway?
Tumor necrosis factor (TNF) receptor ligands (ie, TNF-α), FAS receptor ligands (ie, FasL)
What are the key steps in the intrinsic pathway of apoptosis?
Loss of anti-apoptotic molecules (ie, Bcl-2) and gain of pro-apoptotic molecules (ie, Bak, Bax, Bim) in the mitochondrial membrane, increased mitochondrial membrane permeability and release of cytochrome c, activation of caspase-9
What are the key steps in the extrinsic pathway of apoptosis?
Creation of a death domain by ligand binding of TNFR1 or FAS and adaptor proteins. The death domain then cleaves and activates pro-caspase 8 (ie, creating caspase 8).
What are the key steps in the cytotoxic T-lymphocyte (CTL) mediated pathway of apoptosis?
CTLs secrete perforin allowing entry of granzyme B and activation of executioner caspases. CTLs also secrete Fas ligand to initiate the extrinsic apoptotic pathway.
What are the key steps in the execution phase of apoptosis?
Activated caspase-9 or activated caspase-8 lead to cascade and activation of caspase-3 and/or caspase-6 (executioner caspases), disruption of cytoskeletal components or cell replication machinery, and changes to cell surface molecules which facilitates phagocytosis.
What are the histologic features of necrosis?
Increased cytoplasmic eosinophilia, vacuolated cytoplasm, nuclear changes (karyolysis, pyknosis, or karyorrhexis), calcification, and inflammation in adjacent tissue
Give examples of histologic patterns of necrosis:
Liquefactive necrosis, coagulative necrosis, and caseous necrosis
In which type of necrosis is normal tissue architecture rapidly transformed into a liquid mass due to autolysis and heterolysis?
Liquefactive necrosis
Give examples of liquefactive necrosis:
Pancreatitis, bacterial abscess, central nervous system (CNS) infarction, gastric ulcer, and fungal infection
What is the common pattern of necrosis observed in ischemic and infracted tissue?
Coagulative necrosis, except for CNS ischemia/infarction
Describe the appearance of coagulative necrosis:
The tissue has a firm texture, general tissue architecture is maintained, and “ghost” outlines of necrotic, anucleated cells may be present for weeks before undergoing phagocytosis.
Describe the appearance of caseous necrosis:
The tissue has a soft “cheesy” appearance, general tissue architecture is obliterated, and “ghost” outlines of anucleated cells may be present.
When is caseous necrosis likely?
Tuberculosis or fungal infection and at the center of malignant tumors
Define fat necrosis:
Fat degradation with possible saponification most commonly due to release of enzymes from the pancreas
When does one see fat necrosis?
Acute pancreatitis, ruptured ulcer, penetrating trauma, and subcutaneous infection
A synonym of gangrenous necrosis which is generally used to describe a limb which has lost blood supply and undergone coagulative necrosis
Define dystrophic calcification:
Local deposition of predominately calcium salts in injured or necrotic tissue in the setting of otherwise normal calcium levels
Define metastatic calcification:
Local or wide deposition of predominately calcium salts in otherwise normal tissue in the setting of hypercalcemia
What are the histologic features of dystrophic and metastatic calcification?
Amorphous, basophilic granules in intracellular, extracellular, or in both locations. Over time, ossification may occur at sites of dystrophic calcification.
Define hypoxia:
A state of reduced oxygen availability (ie, poor hemoglobin saturation, inadequate ventilation, hemolysis)
Define ischemia:
A state of significantly reduced blood flow (ie, thrombotic occlusion, trauma), which leads to tissue damage if not reversed
What are the early consequences of ischemic injury?
Transient shift to anaerobic glycolysis; disturbed ionic and fluid balance; inhibited beta-oxidation of fatty acids
What are the late consequences of ischemic injury?
Lysosomal activation; leakage of proteins into serum (creatine kinase [CK], troponin, myoglobin, cellular enzymes)
Which injures tissues faster, ischemia or hypoxia?
Ischemia. In hypoxic tissues, anaerobic glycosis can continue whereas in ischemic tissues anaerobic glycolysis stops when substrates are depleted or when there is accumulation of excessive waste products due to the impaired blood flow.
Why does reperfusion injury occur?
When blood flow is restored, cells that survived the initial ischemia may now be damaged or irreversibly injured by processes initiated by oxygen free radicals, inflammatory cells, or activation of the complement pathway.
What is the process by which lysosomes digest material from the extracellular environment?
Heterophagy
What is autophagy?
Lysosomal digestion of a cell’s own components
What are the three types of intracellular accumulations?
- Excess of a normal cellular constituent
- Abnormal substance
- Pigments (exogenous or endogenous)
How do intracellular accumulations of protein appear?
Generally as discrete eosinophilic cytoplasmic droplets, vacuoles, or aggregates
Which cell type scavenges for exogenous pigments?
Macrophages
What is the most common exogenous pigment?
Carbon or coal
When a person gets a tattoo, where does the pigment go?
The pigment is ingested by dermal macrophages, usually without an inflammatory response.
Give examples of endogenous pigments:
Lipofuscin, hemosiderin, melanin, hematin, bilirubin
Iron is stored in cells in the form of which pigment?
Hemosiderin
What color does hemosiderin stain?
Blue with the Prussian blue histochemical stain and yellow-brown with hematoxylin-eosin (H&E) stain
What pigment is derived from hemoglobin but contains no iron?
Bilirubin
Define jaundice:
Excess of bilirubin within cells and tissues
What is the only endogenous brown-black pigment and how is it formed?
Melanin; it is formed during the oxidation of tyrosine to dihydroxyphenylalanine (DOPA) by tyrosinase in melanocytes.
Define inflammation:
Biologic response to a perceived injurious agent that results in vascular changes which allow fluid and leukocytes into extravascular tissue.
Early and immediate response to injury lasting for a short duration
What features and cell type typically characterize the acute phase of inflammation?
Hyperemia (rubor), pain (dolor), heat (calor), edema/swelling (tumor); polymorphonuclear (PMN) leukocytes
What is edema?
Excess transudative or exudative fluid in the interstitial space or a body cavity
Define transudate:
A clear, extravascular, low-protein, low-cellularity fluid usually due to changes in hydrostatic or osmotic pressure. Specific gravity is <1.012.
Define exudate:
A clear to cloudy extravascular, high protein, high-cellularity fluid usually due to changes in capillary permeability. Specific gravity is >1.012.
What is the term for an exudate rich in neutrophils and parenchymal cell debris?
Pus (purulent exudate)
What is the term for an acute inflammatory process where there is an overlay of fibrin and debris on a mucous membrane?
Pseudomembrane formation
What are the key steps in acute inflammation?
Vasodilation, increased vascular permeability and exudation into extravascular tissues, intravascular stasis, and leukocyte margination
What causes vasodilation and increased vascular permeability in acute inflammation?
Inflammatory mediators such as histamine, nitric oxide, bradykinin, interleukin-1, tumor necrosis factor, and interferon-γ
Which two vasoactive amines are among the first mediators to be released in acute inflammation? Why?
- Histamine
- Serotonin
These are the first to be released because they are present in preformed stores in mast cells, basophils, and/or platelets.
What are the steps of leukocyte extravasation?
- Margination, rolling, and adhesion to endothelium
- Transmigration across endothelium (leukocyte diapedesis)
- Migration toward a chemotactic stimulus in tissues
Which cell surface molecule families play a role in leukocyte adhesion?
Selectins (P-selectin, E-selectin on endothelial cells, and L-selectin on leukocytes), immunoglobulins (ICAM-1 and VCAM-1 on endothelial cells), and integrins (on leukocytes)
What are the common chemotactic agents?
Bacterial products, complement (especially C5a), leukotrienes, cytokines (interleukin-8)
What is the major pathway by which chemotactic agents cause leukocyte movement?
Chemotactic agents bind seven-transmembrane G-protein-coupled receptors leading to activation of effector and second messenger molecules which ultimately induced cytoskeleton component polymerization and contraction.
What are the three stages of phagocytosis?
- Recognition and attachment
- Engulfment
- Killing and degradation
What enzymes or molecules are involved in O2-dependent phagocytosis?
Nicotinamide adenosine dinucleotide phosphate (NADPH) oxidase, H2O2 activity, superoxide radicals, NADPH oxygenase
What enzymes or molecules are involved in O2-independent phagocytosis?
BPI (bactericidal permeability increasing protein), lactoferrin, lysozyme, major basic proteins, defensins
What is the role of the complement system in inflammation?
The complement system is a part of the innate and adaptive immune system by contributing to mediation of vascular permeability and vasodilation, leukocyte adhesion and chemotaxis, and phagocytosis.
Which complement cleavage product is a powerful chemoattractant?
C5a
Which two components of the complement system act as opsonins to coat bacteria?
- C3b
- iC3b
*“Be covered”
What is the role of the kinin system in inflammation?
The kinin system serves to produce bradykinin which mediates vascular permeability and vasodilation.
Which neutrophil storage structure may contain lactoferrin, lysozyme, and collagenase?
Specific granules
*Think Specific are Smaller than azurophil
Which neutrophil storage structure may contain myeloperoxidase, def ensins, and elastases?
Azurophil granules
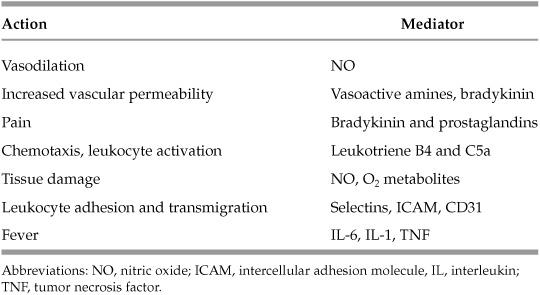
Table 1.1 Mediator Associations
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


