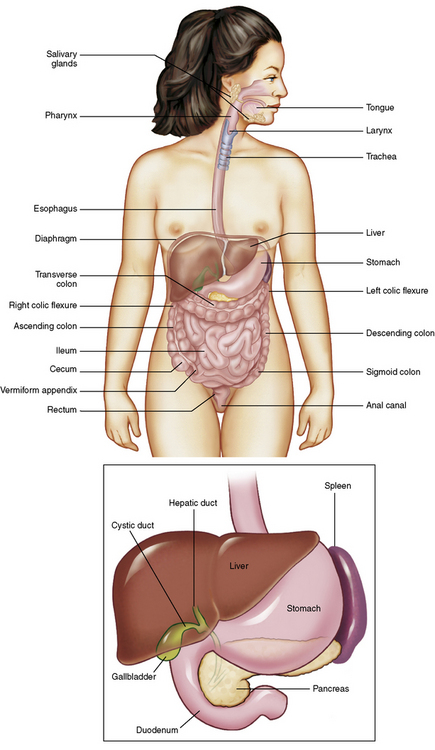
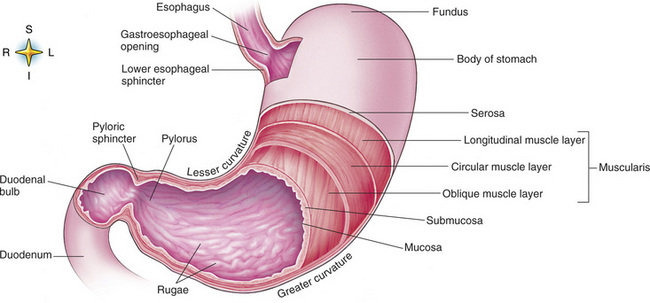
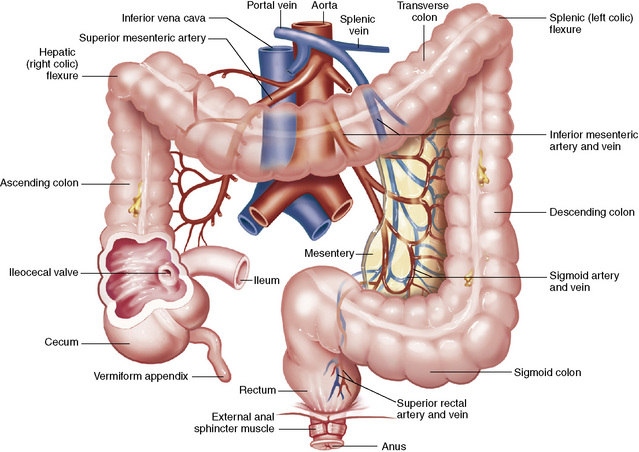
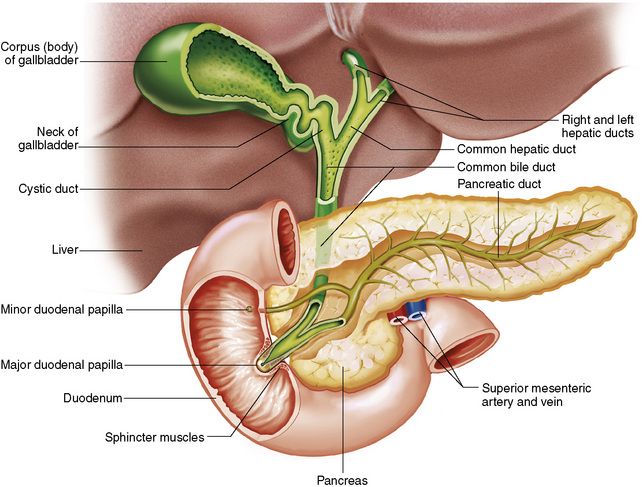
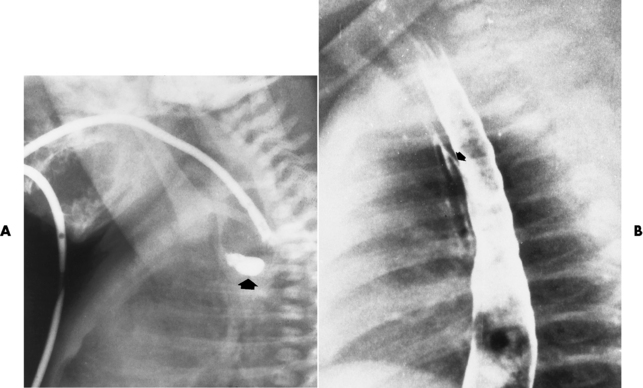
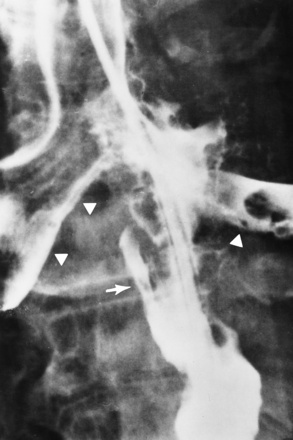
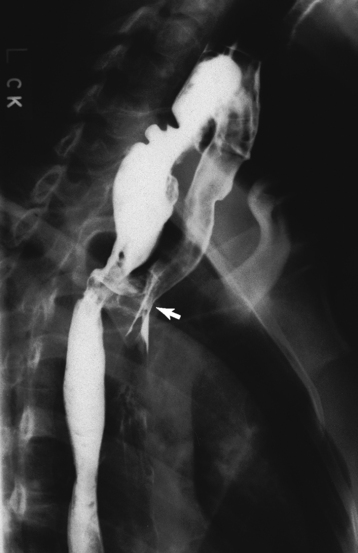
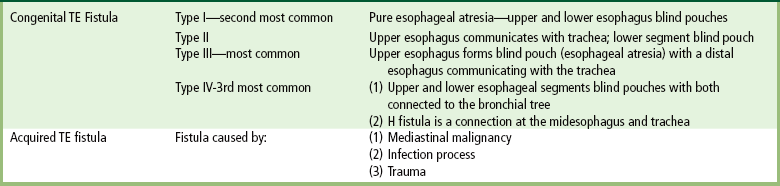
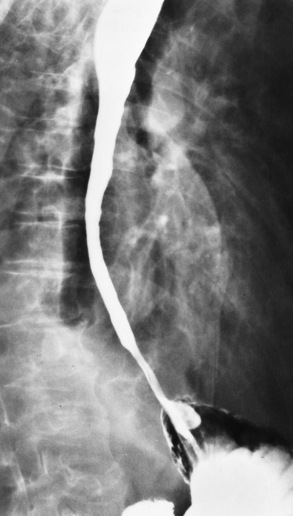
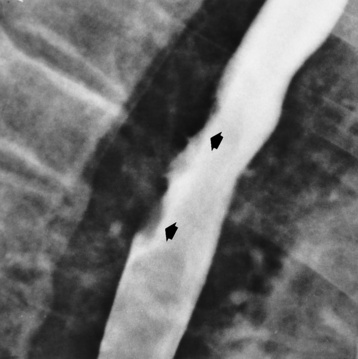
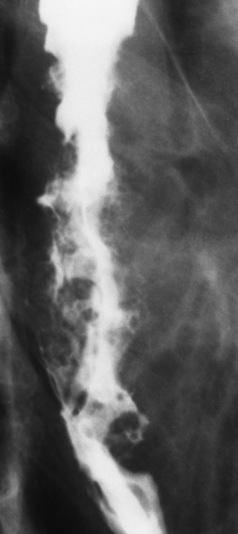
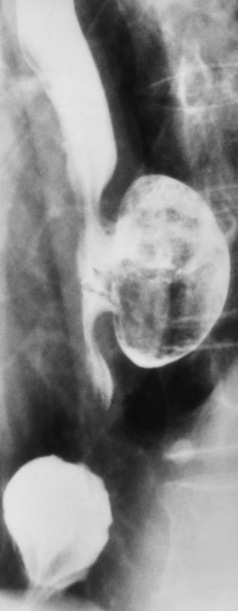
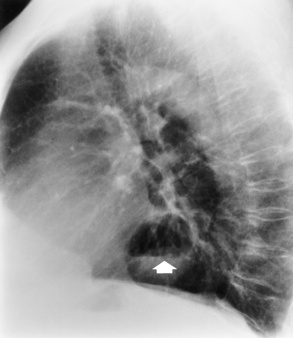
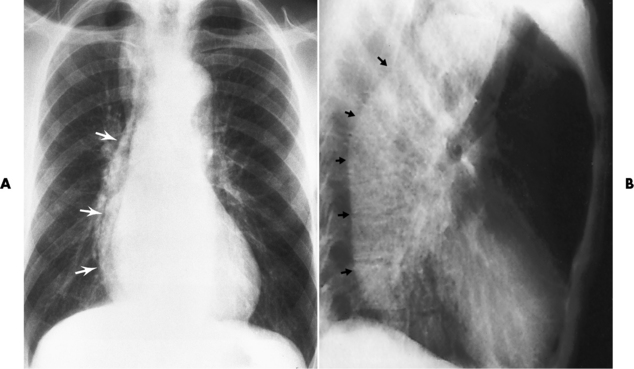
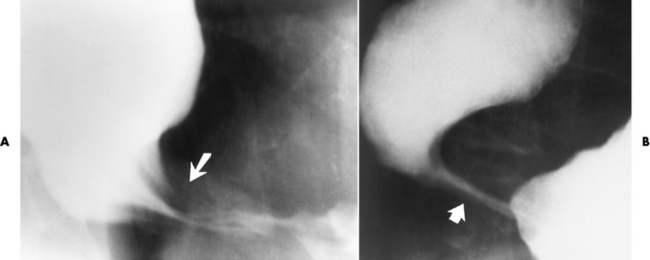
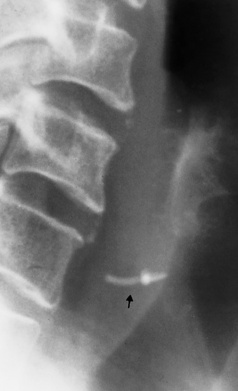
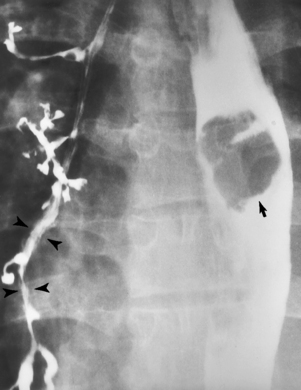
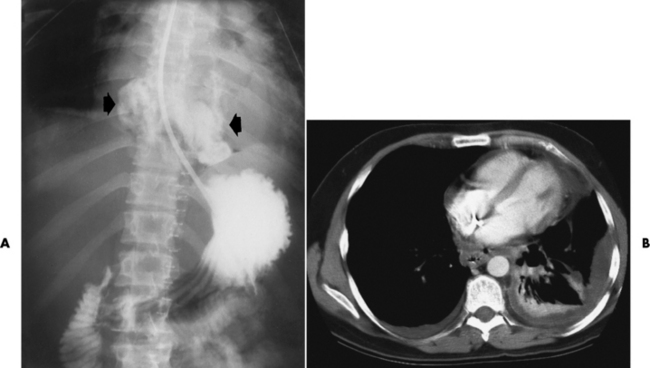
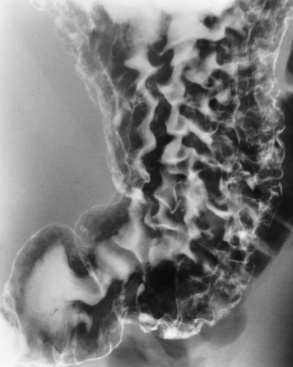
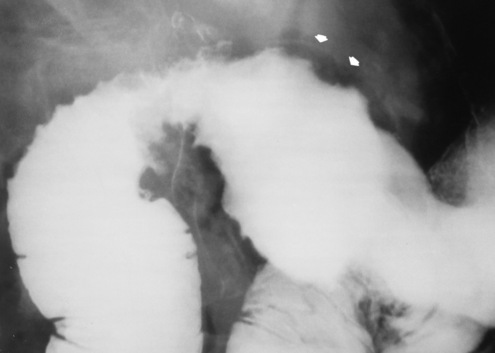
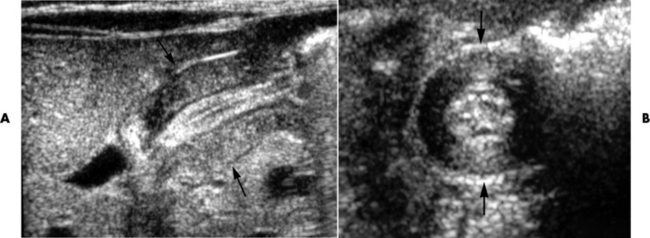
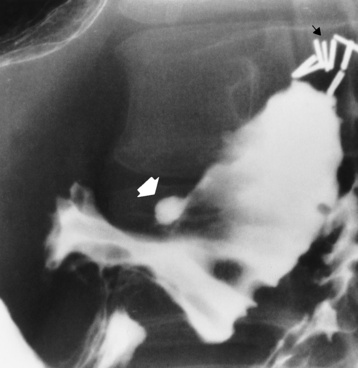
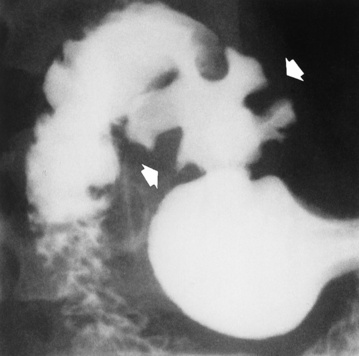
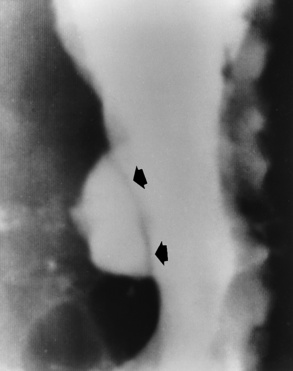
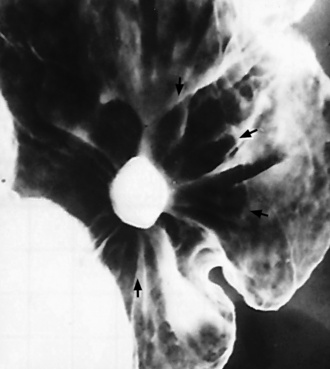
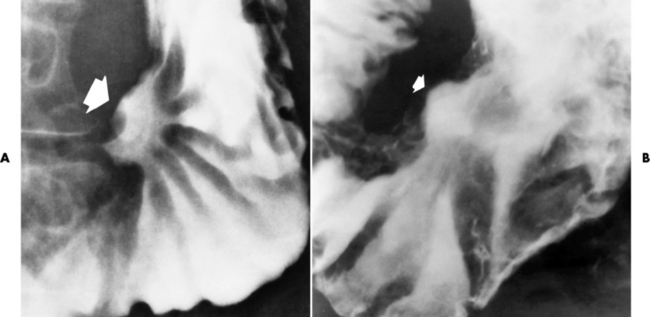
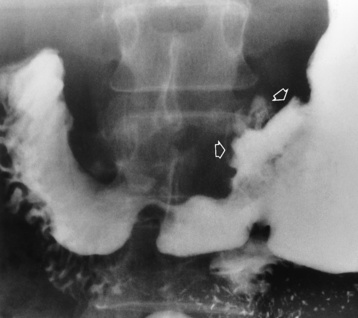
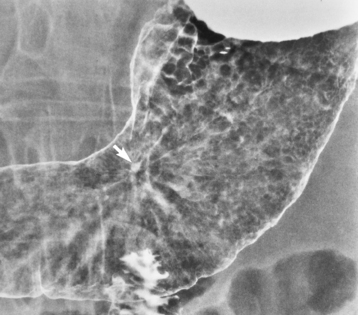
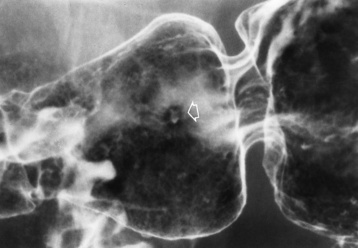
Chapter 5 After reading this chapter, the reader will be able to: 1 Classify the more common diseases in terms of their attenuation of x-rays 2 Explain the changes in technical factors required for obtaining optimal quality radiographs in patients with various underlying pathologic conditions 3 Define and describe all bold-faced terms in this chapter 4 Describe the physiology of the gastrointestinal system 5 Identify anatomic structures on both diagrams and radiographs of the gastrointestinal system 6 Differentiate the various pathologic conditions affecting the gastrointestinal system and their radiographic manifestations The basic function of the digestive system is to alter the chemical and physical composition of food so that it can be absorbed and used by body cells. This process depends on secretions of the endocrine and exocrine glands and on the controlled movement of ingested food through the tract so that absorption can occur (Figure 5-1). Digestion begins in the mouth with chewing (mastication), the mechanical breakdown of food. The secretion of saliva moistens the food in preparation for swallowing. Swallowing (deglutition) is a complex process that requires coordination of many muscles in the head and neck and the precise opening and closing of esophageal sphincters. Digestion continues in the stomach with the churning movement of gastric contents that have become mixed with hydrochloric acid and the proteolytic enzyme pepsin (Figure 5-2). The resulting milky white chyme is propelled through the pyloric sphincter into the duodenum by rhythmic smooth muscle contractions called peristalsis. The greatest amount of digestion occurs in the duodenum, the first part of the small bowel. In addition to intestinal secretions containing mucus and enzymes, secretions of the pancreas and liver enhance digestion in this region. The pancreas secretes enzymes for the digestion of proteins (trypsin and chymotrypsin), fat (lipase), and carbohydrates (amylase). It also secretes an alkaline solution to neutralize the acid carried into the small intestine from the stomach. Bile is secreted by the liver, is stored in the gallbladder, and enters the duodenum through the common bile duct. Bile is an emulsifier, a substance that acts like soap by dispersing the fat into very small droplets that permit it to mix with water. When digestion is complete, the nutrients are absorbed through the intestinal mucosa into blood capillaries and lymph vessels of the wall of the small bowel. The inner surface area of the small bowel is increased by the formation of numerous finger-like projections (villi), which provide the largest amount of surface area possible for digestion and absorption. Material that has not been digested passes into the colon, where water and minerals are absorbed, and the remaining matter is excreted as feces (Figure 5-3). If the contents of the lower colon and rectum move at a rate that is slower than normal, extra water is absorbed from the fecal mass to produce a hardened stool and constipation. Diarrhea results from increased motility of the small bowel, which floods the colon with an excessive amount of water that cannot be completely absorbed. Liver cells play a vital role in the metabolism of proteins, fats, and carbohydrates. The liver is the major site of synthesis of the enzymes necessary for various cellular activities throughout the body. Liver cells also synthesize blood proteins, such as albumin, which maintains the correct amount of fluid within blood vessels, and the essential proteins required for blood clotting (fibrinogen and prothrombin). Therefore, liver damage may result in edema (excess water in the soft tissues) and a serious bleeding tendency. The liver plays an important role in maintaining the proper level of glucose in the blood by taking up excess glucose absorbed by the small intestine and storing it as glycogen. When the level of circulating glucose falls below normal, the liver breaks down glycogen and releases glucose into the bloodstream. Liver cells also store iron and vitamins A, B12, and D. The gallbladder is a pear-shaped sac that lies on the undersurface of the liver (Figure 5-4). Its function is to store bile that enters by way of the hepatic and cystic ducts and to concentrate the bile by absorbing water. In response to the presence of dietary fat in the small bowel, the gallbladder contracts and ejects the concentrated bile into the duodenum. Congenital tracheoesophageal (TE) fistulas result from the failure of a satisfactory esophageal lumen to develop completely separate from the trachea. The lack of the development of the esophageal lumen resulting in a blind pouch describes congenital esophageal atresia. Esophageal atresia and TE fistulas are often associated with other congenital malformations involving the skeleton, cardiovascular system, and GI tract. Radiographic Appearance: In the second most common type of esophageal anomaly, type I, both the upper and lower segments of the esophagus are blind pouches. This anomaly can be differentiated from the type III lesion (the most common type) only by plain abdominal radiographs, which demonstrate the absence of air below the diaphragm in the type I lesion and the presence of air below the stomach in the type III lesion. The type III TE fistula (seen in 85% to 90% of cases) consists of an upper segment that ends in a blind pouch at the level of the bifurcation of the trachea or slightly above it, and a lower segment attached to the trachea by a short fistulous tract. Radiographic demonstration of the looping of a small esophageal feeding tube indicates that the proximal esophagus ends in a blind pouch (Figure 5-5A). Plain radiographs of the abdomen demonstrate the presence of air in the bowel that has freely entered the stomach through the fistulous connection between the trachea and the distal esophagus. There are two forms of type IV TE fistula. In one, the upper and lower esophageal segments end in blind pouches, both of which are connected to the tracheobronchial tree. In this form, gas is seen in the stomach, and oral contrast material outlines both fistulas and the bronchial tree. In the other form of type IV TE fistula (called an H fistula), both the trachea and the esophagus are intact. These two structures are connected by a single fistulous tract that can be found at any level from the cricoid cartilage of the trachea to the tracheal bifurcation (Figure 5-5B). Unlike the other forms of TE fistula, the H fistula may not be identified in infancy and, if it is small and only occasionally causes emptying of material into the lungs, can permit survival into adulthood. Fistulization between the esophagus and the respiratory tract is a major late complication of esophageal carcinoma and is often a terminal event (Figure 5-6). A fistula can also be a complication of erosion into the esophagus either by carcinoma of the lung arising near or metastasizing to the middle mediastinum or by mediastinal metastases from other primary sites. Regardless of therapy, the overall prognosis of malignant TE fistulas is dismal, and more than 80% of patients with this complication die within 3 months from uncontrollable hemorrhage or from pulmonary infection caused by repeated episodes of aspiration pneumonia. Fistulous communications between the esophagus and the tracheobronchial tree can be the result of esophageal instrumentation and perforation (Figure 5-7). It is most common after esophagoscopy but may also occur after instrumental dilation of strictures by bougienage, pneumatic dilation of the esophagus for the treatment of achalasia, or even the insertion of a nasogastric tube. Blunt or penetrating trauma to the chest, especially after crush injury, can result in esophageal perforation and fistulization. Radiographic Appearance: After traumatic perforation of the thoracic esophagus, chest radiographs may demonstrate air dissecting within the mediastinum and soft tissue, often with pleural effusion or hydropneumothorax. The introduction of an oral contrast agent may demonstrate the site of perforation and the extent of fistulization. Reflux (Gastroesophageal Reflux Disease) inflammatory response. Gastroesophageal reflux disease (GERD) describes any symptomatic condition or structural changes caused by reflux of the stomach contents into the esophagus. Alcohol, chocolate, caffeine, and fatty foods tend to decrease the pressure of the esophageal sphincter, allowing reflux to occur. Regardless of the cause, acute esophagitis produces burning chest pain that may simulate the pain of heart disease. Superficial ulcerations are most typical of reflux. The esophagus is often dilated, with a loss of effective peristalsis. Nonpropulsive peristaltic waves, ranging from mild tertiary contractions to severe segmental spasms, are an early finding. Radiographic Appearance: The earliest radiographic findings in reflux esophagitis are detectable on double-contrast studies. They consist of superficial ulcerations or erosions that appear as streaks or dots of barium superimposed on the flat mucosa of the distal esophagus. In single-contrast studies of patients with esophagitis, the outer borders of the barium-filled esophagus are not sharply seen but rather have a hazy, serrated appearance with shallow, irregular protrusions indicating erosions of varying length and depth. Widening and coarsening of edematous longitudinal folds can simulate filling defects. In addition to diffuse erosion, reflux esophagitis can result in large, discrete, penetrating ulcers in the distal esophagus (Figure 5-8) or in a hiatal hernia sac (Figure 5-9). Fibrotic healing of diffuse reflux esophagitis or a localized penetrating ulcer may cause narrowing of the distal esophagus. Strictures resulting from reflux esophagitis tend to be smooth and tapering with no demonstrable mucosal pattern (Figure 5-10). Radiographic Appearance: Although a hiatal hernia with gastroesophageal reflux is commonly demonstrated, Barrett’s ulcer is usually separated from the hiatal hernia by a variable length of normal-appearing esophagus (Figure 5-11), in contrast to reflux esophagitis, in which the distal esophagus is abnormal down to the level of the hernia. As in reflux esophagitis, fibrotic healing of the ulceration in Barrett’s esophagus often leads to a smooth, tapered stricture (Figure 5-12). Figure 5-11 Barrett’s esophagus. Ulcerations (arrow) have developed at a distance from esophagogastric junction. Figure 5-12 Barrett’s esophagus. Note the smooth, tapered stricture in the upper thoracic esophagus. Radiographic Appearance: The classic radiographic appearance of infectious esophagitis is an irregular cobblestone pattern with a shaggy marginal contour of the esophagus caused by deep ulcerations and sloughing of the mucosa (Figure 5-13). Candida infection manifests as plaques and nodules resulting from a superficial collection of fungi. Characteristics of herpetic esophagitis include small mucosal ulcers or plaques. Radiographic Appearance: Healing of the intense mucosal and intramural inflammation of acute esophagitis may lead to pronounced fibrosis and stricture formation. These benign strictures tend to be long lesions with tapered margins and relatively smooth mucosal surfaces (Figure 5-14), in contrast to the irregular narrowing, mucosal destruction, and overhanging margins that are generally associated with malignant processes. Treatment: The type of agent ingested determines the therapy. The local or state poison control service is usually called for specific treatments if the situation is not drug induced. Vomiting is generally not induced because this would cause a second exposure of the esophagus to the agent. Dilution by administration of milk or water is appropriate unless the corrosive agent is acidic (in this case, water should not be used as it would produce excessive heat). The earliest radiographic evidence of infiltrating carcinoma of the esophagus appears on a double-contrast barium swallow image as a flat, plaquelike lesion, occasionally with central ulceration, that involves one wall of the esophagus (Figure 5-15). At this stage, there may be minimal reduction in the caliber of the lumen. Unless the patient is carefully examined in various positions, this earliest form of esophageal carcinoma can be missed. As the infiltrating cancer progresses, irregularity of the wall is seen, indicating mucosal destruction. Advanced lesions encircle the lumen completely, causing annular constrictions with overhanging margins and often some degree of obstruction. The lumen through the stenotic area is irregular, and mucosal folds are absent or severely ulcerated (Figure 5-16). Less commonly, carcinoma of the esophagus can appear as a localized polypoid mass, often with deep ulceration and a fungating appearance. Luminal obstruction as a result of carcinoma causes proximal dilation of the esophagus and may result in aspiration pneumonia. Extension of the tumor to adjacent mediastinal structures may lead to fistula formation, especially between the esophagus and the respiratory tract (see Figure 5-20). Wall thickening greater than 3 to 5 mm on a CT scan is suggestive of esophageal cancer. CT has become a major method of staging patients with esophageal carcinoma (with 90% accuracy), providing information on tumor size, extension, and resectability that was previously available only at thoracotomy (Figure 5-17). Evidence of tumor spread includes the obliteration of fat planes between the esophagus and adjacent structures (left atrium, aorta), the formation of a fistula to the tracheobronchial tree, and recognition of metastatic disease (e.g., low-density masses in the liver, enlargement of draining lymph nodes). Use of contrast enhancement improves the detail of tumor delineation. Zenker’s diverticula arise from the posterior wall of the upper (cervical) esophagus (Figure 5-18). Occasionally, they can become so large that they almost occlude the esophageal lumen. CT prominently demonstrates the cricopharyngeal muscle, which aids in locating the origin of Zenker’s diverticula at the pharyngoesophageal junction. Diverticula of the thoracic portion of the esophagus are primarily found opposite the bifurcation of the trachea, in the region of the hilum of the lung (Figure 5-19). These traction diverticula reflect motor function disturbance and develop in response to the pull of fibrous adhesions after infection of the mediastinal lymph nodes. Epiphrenic diverticula arise in the distal 10 cm of the esophagus (Figure 5-20). They are associated with incoordination of esophageal peristalsis and sphincter relaxation, which increases the intraluminal pressure in this segment. The characteristic radiographic appearance of esophageal varices is serpiginous (wavy border) thickening of folds, which appear as round or oval filling defects resembling the beads of a rosary (Figure 5-21). Precise technique is required to demonstrate esophageal varices. A double-contrast barium swallow study best demonstrates the serpiginous and wormlike filling defect. Complete filling of the esophagus with barium may obscure varices, and powerful contractions of the esophagus may squeeze blood out of the varices and make them impossible to detect. Upright and recumbent imaging may best demonstrate the varices dilated and empty, respectively. Figure 5-21 Esophageal varices. Note the diffuse round and oval filling defects, which resemble rosary beads. Although the diagnosis of hiatal hernia generally requires a barium study (Figure 5-22), at times a large hiatal hernia may appear on plain chest radiograph as a soft tissue mass in the posterior mediastinum, often containing a prominent air-fluid level (Figure 5-23). The esophagus and stomach are distinguished by their appearance; mucosal folds are linear and parallel in the esophagus, whereas in the stomach the folds appear numerous and thicker without a parallel orientation. On plain chest radiographs, the dilated, tortuous esophagus may produce a widened mediastinum (often with an air-fluid level) on the right side adjacent to the cardiac shadow (Figure 5-24). The hallmark of achalasia, seen on barium studies, is a gradually tapered, smooth, conical, 1- to 3-cm narrowing of the distal esophageal segment (rat-tail or beak appearance) (Figure 5-25). On sequential radiographs, especially with the patient upright, only small spurts of barium are seen to pass through the narrowed distal segment to enter the stomach. A wide spectrum of foreign bodies can become impacted in the esophagus, usually in the cervical esophagus at or just above the level of the thoracic inlet (Figure 5-26). Symptomatically, the patient is unable to swallow without regurgitation. Most metallic objects, such as pins, coins, and small toys, are radiopaque and are easily visualized on radiographs or during fluoroscopy. Objects made of aluminum and some light alloys may be impossible to detect radiographically because the density of these metals is almost equal to that of soft tissue. It is essential that any suspected foreign body be evaluated on two projections to be certain that the object projected over the esophagus truly lies within it. Nonopaque foreign bodies in the esophagus, especially pieces of poorly chewed meat (masticated food bolus), can be demonstrated only after the ingestion of barium (Figure 5-27). Such a foreign body usually becomes impacted in the distal esophagus just above the level of the diaphragm and is often associated with a distal stricture. The intraluminal filling defect usually has an irregular surface and may resemble a completely obstructing carcinoma. Perforation of the esophagus may be a complication of esophagitis, peptic ulcer, neoplasm, external trauma, or instrumentation. At times, perforation of a previously healthy esophagus can result from severe vomiting (the most common cause) or coughing, often from dietary or alcoholic indiscretion. Complete rupture of the wall of the esophagus may cause the sudden development of severe upper gastric pain simulating that of myocardial infarction. In the Mallory-Weiss syndrome, an increase in intraluminal and intramural pressures associated with vomiting (severe retching) after an alcoholic bout causes superficial mucosal laceration or fissures near the esophagogastric junction that produce severe hemorrhage. Endoscopy is required to best demonstrate lacerations, especially those close to the sphincter. A perforation that extends throughout the entire esophageal wall can lead to free air in the mediastinum or periesophageal soft tissues. The administration of radiopaque contrast material may demonstrate extravasation through the perforation (Figure 5-28) or an intramural dissection channel separated by an intervening lucent line from the normal esophageal lumen. CT is the preferred modality to define the extent of the process. Alcoholic gastritis may produce thickening of gastric folds (Figure 5-29), multiple superficial gastric erosions, or both. In corrosive gastritis, the acute inflammatory reaction heals by fibrosis and scarring, which result in severe narrowing of the antrum and may cause gastric outlet obstruction. In bacterial (phlegmonous) gastritis, inflammatory thickening of the gastric wall causes narrowing of the stomach that may mimic gastric cancer. The diagnosis of infectious gastritis can be made if there is evidence of gas bubbles (produced by the bacteria) in the stomach wall (Figure 5-30). These types of gastritis are known as erosive or acute gastritis. Chronic atrophic gastritis (nonerosive) refers to severe mucosal atrophy (wasting) that causes thinning and a relative absence of mucosal folds, with the fundus or entire stomach having a bald appearance. This is a nonspecific radiographic pattern that can be related to such factors as age, malnutrition, medication, and complications of alcoholism. Chronic atrophic gastritis also occurs in patients with pernicious anemia, who cannot absorb vitamin B12 because of an inability of the stomach to secrete intrinsic factor (or hydrochloric acid). Pyloric stenosis, also known as infantile hypertrophic pyloric stenosis (IHPS), occurs when the two muscular layers of the pylorus become hyperplastic and hypertrophic. Environmental and hereditary factors are believed to cause this process in 2 to 4 per 1000 live births. The gastric antrum and the pyloric canal become lengthened, whereas the mucosa is usually edematous and thickened. This causes a complete or near-complete obstruction, preventing food from entering into the duodenum. The edematous and thickened pylorus may be palpated and is described as a mobile, hard “olive.” In today’s imaging arena, ultrasound is the modality of choice due to its high sensitivity and specificity, an accuracy approaching 100%. Pyloric stenosis appears as a thickened pyloric muscle (width greater than 3 mm) and an elongated pyloric canal (greater than 1.2 cm) on the longitudinal sonogram (Figure 5-31). The palpable olive appears as a “doughnut” or “target” sign in the cross-sectional image. When ultrasonographic findings are inconclusive, an upper GI series may aid in confirming the diagnosis by demonstrating the shouldering caused by a filling defect at the antrum as a result of the hypertrophic pyloric sphincter and delayed gastric emptying. Radiographic Appearance: An unequivocal diagnosis of active duodenal ulcer requires the demonstration of an ulcer crater, which appears in profile as a small collection of barium projecting from the lumen. When seen en face (face on), the ulcer niche appears as a rounded or linear collection of contrast material surrounded by lucent folds that often radiate toward the crater (Figure 5-32). Secondary signs of duodenal ulcer disease include thickening of the mucosal folds and a deformity of the duodenal bulb. Acute ulcers incite muscular spasm, leading to deformity of the margins of the duodenal bulb that may be inconsistent and varied during the examination. With chronic ulceration, fibrosis and scarring cause a fixed deformity that persists even though the ulcer heals. Symmetrical narrowing of the duodenal bulb in its midportion may produce the typical cloverleaf deformity of chronic duodenal ulcer disease (Figure 5-33). CT demonstrates an irregularity or collection of contrast material in the gastric wall; however, as with barium studies, this appearance may be difficult to differentiate from that of malignancy. Radiographic Appearance: Radiographic signs that indicate whether a gastric ulcer is more likely to be benign or malignant have been described. The classic sign of a benign gastric ulcer in profile is penetration, with clear projection of the ulcer outside the normal barium-filled gastric lumen because the ulcer represents an excavation in the wall of the stomach (Figure 5-34). A thin lucency at the base of the ulcer, reflecting mucosal edema caused by inflammatory exudate, is another sign of benignancy. When viewed en face, a gastric ulcer appears as a persistent collection of barium surrounded by a halo of edema (the ulcer collar) (Figure 5-35). A hallmark of benign gastric ulcer is radiation of mucosal folds to the edge of the crater. However, because radiating folds can be identified in both malignant and benign ulcers, the character of the folds must be carefully assessed. If the folds are smooth and slender and appear to extend into the edge of the crater, the ulcer is most likely benign (Figure 5-36A). In contrast, irregular folds that merge into a mound of polypoid tissue around the crater are suggestive of malignancy (Figure 5-36B). An abrupt transition between the normal mucosa and the abnormal tissues surrounding a gastric ulcer is characteristic of a malignant lesion (Figure 5-37), in contrast to the diffuse and almost imperceptible transition between the normal gastric mucosa and the mound of edema surrounding a benign ulcer. Neoplastic tissue surrounding a malignant ulcer is usually nodular, unlike the smooth contour of the edematous mound around a benign ulcer. A malignant ulcer does not penetrate beyond the normal gastric lumen but remains within it because the ulcer merely represents a necrotic area within an intramural or intraluminal mass. Most benign gastric ulcers heal completely with medical therapy (see “Treatment of Ulcers”) (Figure 5-38). Complete healing does not necessarily mean that the stomach returns to an absolutely normal radiographic appearance; bizarre deformities can result from fibrotic retraction and stiffening of the stomach wall. Although many malignant ulcers show significant healing, there is almost never complete disappearance of the ulcer crater. Superficial gastric erosions are ulcerations that are so small and shallow that they are rarely demonstrated on conventional single-contrast upper GI examinations. With the increasing use of double-contrast techniques, a superficial gastric erosion typically appears radiographically as a tiny fleck of barium, which represents the erosion, surrounded by a radiolucent halo, which represents a mound of edematous mucosa (Figure 5-39). Possible factors implicated in the production of superficial gastric erosions include alcohol, antiinflammatory drugs (aspirin, steroids), Crohn’s disease (see later discussion), and candidiasis (see earlier discussion “Candida and Herpesvirus”).
Gastrointestinal System
Physiology of the digestive system
Esophagus
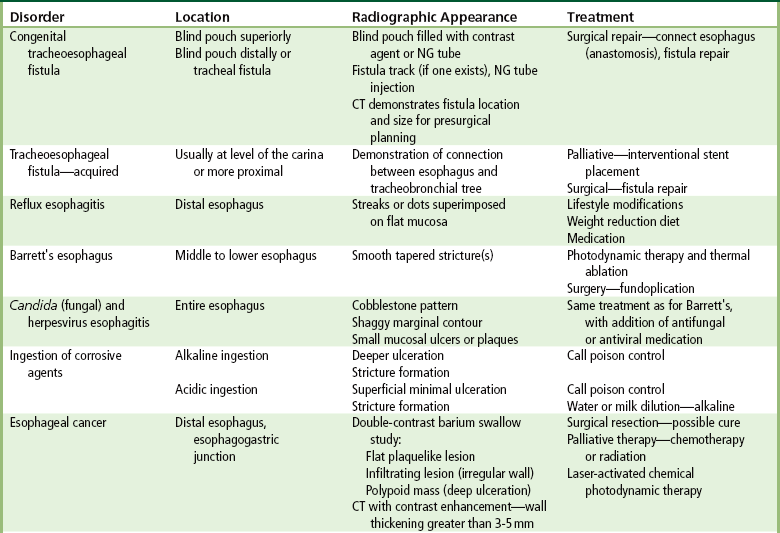
Congenital Type
Acquired Type
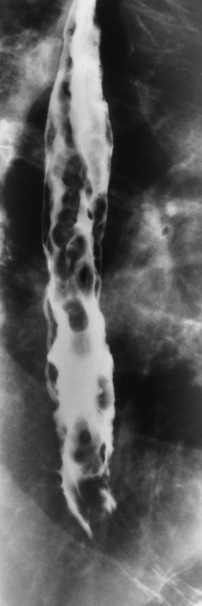
Esophagitis
Barrett’s Esophagus
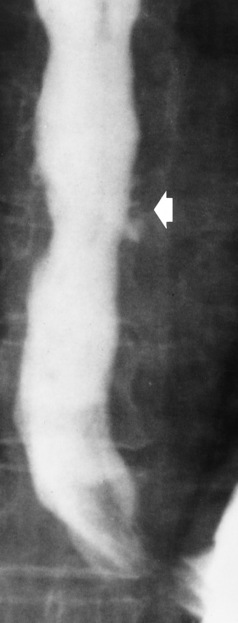
Candida and Herpesvirus
Ingestion of Corrosive Agents
Esophageal Cancer
Radiographic Appearance
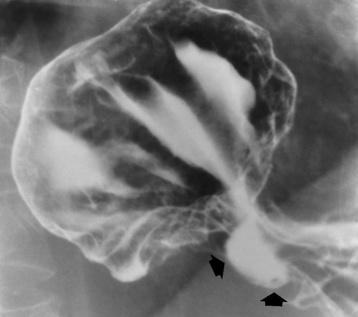
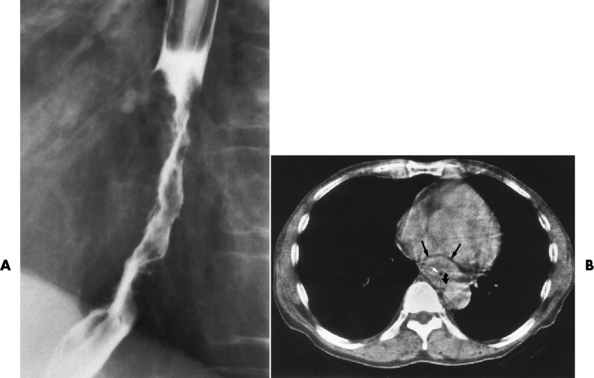
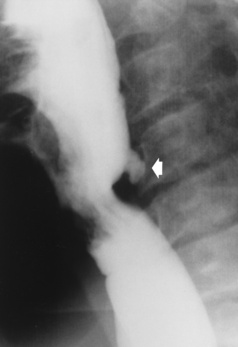
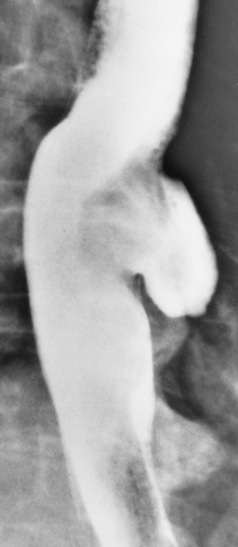
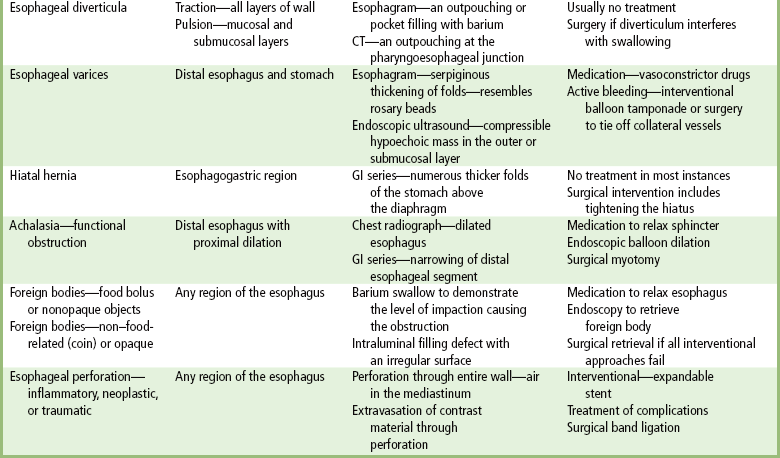
Esophageal Diverticula
Radiographic Appearance
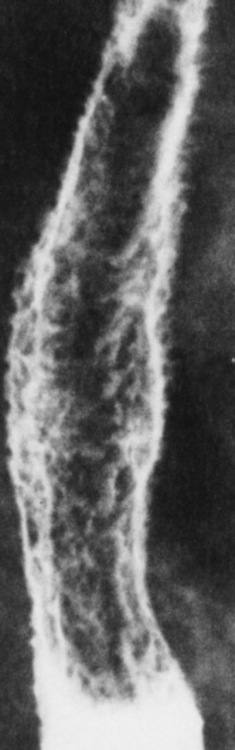
Esophageal Varices
Radiographic Appearance
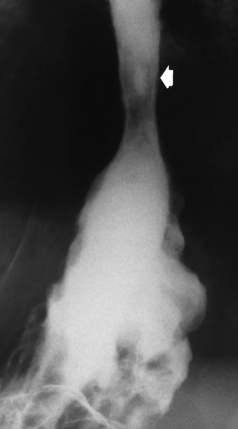
Hiatal Hernia
Radiographic Appearance
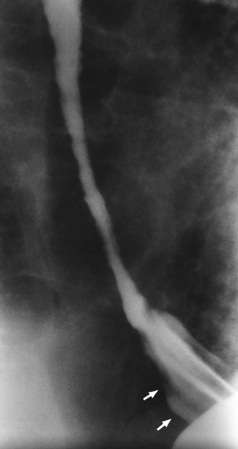
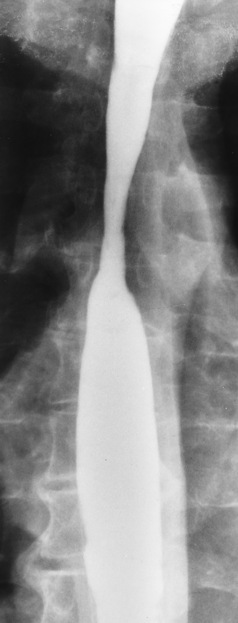
Achalasia
Radiographic Appearance
Foreign Bodies
Radiographic Appearance
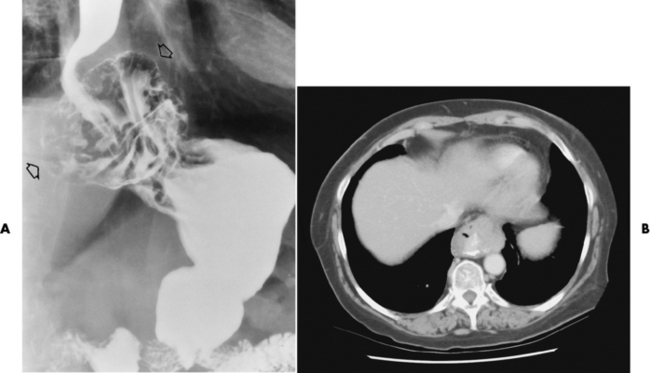
Perforation of the Esophagus
Radiographic Appearance
Stomach
Radiographic Appearance
Pyloric Stenosis
Radiographic Appearance
Peptic Ulcer Disease
Duodenal Ulcer
Gastric Ulcer
Superficial Gastric Erosions
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Gastrointestinal System