Gastrointestinal-Cutaneous Fistulas
Josef E. Fischer
Amy R. Evenson
Enterocutaneous fistulas (ECFs) represent a catastrophic complication of abdominal disease, usually following surgical procedures. At least 85%, and perhaps as many as 95%, follow abdominal operations. Spontaneous fistulas usually occur as a complication of inflammatory bowel disease, cancer, or radiation. Spontaneous fistulas complicating cancer usually occur in advanced disease, and the prognosis, for the most part, is poor. Modern management strategies have resulted in improved overall mortality from upward of 50% to 60% between the 1940s and 1950s to between 10% and 20% from the 1960s through the turn of the century. At present, in the proper hands, mortality may range from only 0% to 2%, usually from complications of sepsis.
When a fistula follows operation, it is usually the result of an unrealized enterotomy or leaking anastomosis, often because of poorly prepared or distended bowel, or emergency operation with the patient nutritionally or immunologically in suboptimal condition. A fistula may also follow inadequate blood supply to the bowel or, in many situations, distended or weakened small bowel due to a delay in relieving partial or near-total intestinal obstruction.
Recently, a new cause has appeared for postoperative fistulas and this is gastrointestinal cutaneous fistulas following open or especially laparoscopic ventral abdominal herniorrhaphy. The fistula seems to be caused by one of two etiologies: the first being an inadvertent enterotomy; the second, and more common, is the use of permanent mesh which may become infected and erode into the bowel, or a fastening tack that may injure the bowel inadvertently causing arcing of an electrical current which may in turn also cause bowel injury. In the case of the infected mesh, it is rare for fistula to close without removing the offending mesh. Rarely, mesh removal alone is sufficient for the fistula to close without bowel resection. Frequently, however, bowel resection is also required. At the present rate of presentation, this may become one of the more common causes of gastrointestinal fistula, as in some series antecedent ventral abdominal herniorrhaphy is responsible for 10% of ECFs.
Etiology and Prevention
Enterocutaneous fistulas result from one of the following several conditions:
Extension of bowel disease to surrounding structures;
Extension of adjacent non-bowel disease to normal bowel;
Inadvertent or unrecognized injury to the bowel; or
Anastomotic disruption.
As stated earlier, 85% to 95% of fistulas occur in the postoperative period. Operation for complications of inflammatory bowel disease, resection of malignancy, adhesiolysis for intestinal obstruction, and herniorrhaphy are the most common antecedent procedures. Preoperative factors may increase the likelihood of development of an ECF. These factors include malnutrition, infection, emergency procedures with preoperative hypotension, anemia, hypothermia, or poor oxygen delivery. In elective procedures, these aggravating factors should be corrected prior to operation with nutritional support, bowel preparation, and control of abnormal physiological
parameters such as cardiac output, hyperglycemia, and anemia. Ideally, serum albumin should be >3.3 g/dL (better yet, 3.5 g/dL) and the patient should not have lost >10% or 15% of body weight in the preceding four months. Diabetes should be controlled, and if time permits, 5 days of parenteral nutrition should be administered. My experience is that within 5 days of adequate nutritional support, the patient begins to feel better and serum transferrin will increase. However, serum albumin will not increase because of the half-life of ∼20 days. Mechanical and antibiotic bowel preparation, if feasible, should be carried out, although some doubt the efficacy of both mechanical and antibiotic bowel preparation. The current view is that it is not harmful except for Clostridia difficile if antibiotics are given orally as a preparation. All agree, however, that the administration of intravenous antibiotics within one-half hour of the incision and maintenance of satisfactory blood antibiotic levels throughout the procedure, which may be prolonged sometimes, are necessary. While this preparation will not obviate the occurrence of fistulas completely, they may diminish their occurrence. Abscesses will be minimized at the very least.
parameters such as cardiac output, hyperglycemia, and anemia. Ideally, serum albumin should be >3.3 g/dL (better yet, 3.5 g/dL) and the patient should not have lost >10% or 15% of body weight in the preceding four months. Diabetes should be controlled, and if time permits, 5 days of parenteral nutrition should be administered. My experience is that within 5 days of adequate nutritional support, the patient begins to feel better and serum transferrin will increase. However, serum albumin will not increase because of the half-life of ∼20 days. Mechanical and antibiotic bowel preparation, if feasible, should be carried out, although some doubt the efficacy of both mechanical and antibiotic bowel preparation. The current view is that it is not harmful except for Clostridia difficile if antibiotics are given orally as a preparation. All agree, however, that the administration of intravenous antibiotics within one-half hour of the incision and maintenance of satisfactory blood antibiotic levels throughout the procedure, which may be prolonged sometimes, are necessary. While this preparation will not obviate the occurrence of fistulas completely, they may diminish their occurrence. Abscesses will be minimized at the very least.
In the patient undergoing an emergency procedure, however, optimization of resuscitation and performance of a technically meticulous procedure may be the only steps the surgeon can take to prevent ECF. Maintenance of oxygenation, warm room temperatures, optimization of cardiac output, and adequate monitoring may improve the outcome. Sound surgical technique includes an adequate incision, good lighting so that one can visualize the procedure completely, adequate mobilization, and the use of healthy bowel with good blood supply for anastomosis with avoidance of tension, hemostasis, and adequate transfusion, which minimizes the risk of formation of an ECF. Additionally, prior to abdominal closure, the bowel should be inspected for inadvertent bowel injury and all enterotomies should obviously be repaired. It is my practice to repair enterotomies when they occur, as they may be difficult to find later. It is my custom to repair serosal injuries with fine nonabsorbable sutures such as 4-0 or 5-0 Prolene, contrary to what some have proposed.
Spontaneous Enterocutaneous Fistulas
Spontaneous ECF most commonly occurs in the setting of inflammation, malignancy, or irradiation. Inflammatory causes of ECF include Crohn’s disease, and, less commonly, ulcerative colitis, peptic ulcer, appendicitis, diverticulitis, pancreatitis, especially when pancreatic necrosis is treated by repeated dressings, and ischemic bowel. In inflammatory bowel disease, ECFs in the patients with Crohn’s disease represent a special concern for the surgeon. Often a fistula in these patients will resolve with nonoperative management only with parenteral nutrition, only to reopen on resumption of enteral feedings. Some of the newer, immunological-type drugs may result in more frequent closure; however, it is not clear that these fistulas once closed will not recur. It is usually my practice to close the fistula non-operatively when possible and then operate on the patient to resect that portion of bowel when infection in the abdominal wall is quiescent. A second important distinction that is pertinent to the patients with Crohn’s disease involves whether the patient’s fistula arises in healthy bowel (following resection of the diseased segment) or from diseased bowel not necessarily following bowel resection. The former type is probably no different than in the patients without inflammatory bowel disease and has a high likelihood of spontaneous closure. The latter, as stated earlier, has a lower rate of nonoperative closure. Patients with this type of ECF may benefit from early resection especially if it can be closed spontaneously prior to operation, as these fistulas tend to reopen.
In the patients with malignancy or following radiation therapy, different tactics may be used because many of these fistulas will not close without resection. In patients with cancer, occurrence of a spontaneous fistula is a grave sign indicating a poor outcome. An understanding of the etiology of an ECF may provide information about the likelihood of successful closure with or without intervention.
It must be remembered that more than one of these predisposing conditions may exist in any patient and the risks are compounded when multiple factors are present.
Recognition of a postoperative ECF follows a distinct pattern. The patient does not do well immediately following the operation. There is a slow course with fever and a prolonged ileus as well as a feeling of malaise. Abdominal distention may also be part of the picture. Most of all is a lack of progression and recovery from the original operation with a low-grade fever sometimes up to 101°F or even 102°F and more abdominal tenderness than one would ordinarily expect if the operation were uneventful. On the fifth or sixth postoperative day, a “wound infection” presents which is then drained; defervescence follows and the patient improves. There is some relaxation on the part of the surgeon as perhaps the patient will now do well. However, within 24 hours, enteric contents drain from the wound and the surgeon knows that one is dealing with a gastrointestinal cutaneous fistula.
At this point there is a decision to be made. There is data from experienced centers that indicate that immediate reoperation before 9 or 10 days postoperatively results in 10% mortality, whereas operation between 10 and 120 days results in 20% mortality. However, I would caution operating on the patient at this time. The operation is not risk-free because the patient is in suboptimal condition (i.e., some of the same conditions are present that contributed to this patient getting a fistula in the first place, which include the following: suboptimal nutritional condition, distended bowel, early obliterative peritonitis, and probably a suboptimal immunological status). Operation at this point may yield another fistula which will make things worse and probably have a higher mortality. A second alternative, and one which I would support, is to accept the fistula, not reoperate, and counsel the patient that one will try nonoperative closure, and that if this does not work, then reoperation after an interval of 4 or 5 months lies ahead. This will be difficult on the patient and his/her family, but this is likely to result in long-term survival. One needs the support and cooperation of the patient and his/her family to be successful.
Nutritional Support
As soon as the fistula has been discovered, one evaluates the patient. It is likely that the patient is nutritionally deficient and intravascularly depleted with multiple electrolyte deficiencies, especially sodium, potassium, phosphate, and magnesium. There is often a lack of intravascular volume as the patient has been third spacing. Bowel distention leads to movement of fluid as well as protein and electrolytes into the extravascular space as well as within distended bowel. These need to be replenished. Given the sophisticated electrolyte monitoring available, it is surprising that these deficiencies in electrolytes occur quite frequently – patients escape from
tight monitoring. In addition to measuring serum electrolytes, one can get an idea of what the composition of fluid lost is by sending some of the fistula fluid for electrolyte determinations (Table 1). This will help maintenance of normal electrolytes in the serum, as one replaces what is being lost.
tight monitoring. In addition to measuring serum electrolytes, one can get an idea of what the composition of fluid lost is by sending some of the fistula fluid for electrolyte determinations (Table 1). This will help maintenance of normal electrolytes in the serum, as one replaces what is being lost.
Table 1 Electrolyte Composition of Body Fluidsa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The patient should be kept NPO and hard candy may be allowed to keep saliva flowing, avoiding sialadenitis. Aggressive restoration of intravascular volume should take place at once, utilizing 5% Albumisol as well as crystalloid. One must be careful as to how much crystalloid is administered, as one does not want to get an edematous patient after resuscitation, so judicious administration of crystalloid is necessary. Supplementation of magnesium, phosphate, and potassium as well as sodium is required to prevent further electrolyte disturbances. Malnutrition that complicates ECF is multifactorial. Postoperative patients have often endured 1 to 2 weeks of starvation with essentially no oral intake prior to recognition of the fistula. As for those patients with spontaneous fistulas, they have inflammatory bowel disease or malignancies which may lead to poor nutrition for some time. Some patients may have limited absorptive areas of the bowel. Further, ECFs are often accompanied by sepsis and inflammation which increase the metabolic needs.
Nutritional Status
Depleted nutritional status interferes with the ability to resuscitate the patient and is one of the principal determinants of mortality, whereas the ability to reverse poor nutritional status improves outcome. In one large series, patient serum albumin level, at first discovery, was a major factor in survival. Patient serum albumin of <2.5 g/dL had an increased mortality, whereas patients with a normal serum albumin level (>3.5 g/dL) suffered no mortality. Hypoalbuminemia may also inhibit wound healing and may lead to bowel dysfunction, complicating efforts to feed the patient enterally. Other serum markers of nutrition which may predict mortality include transferrin, retinol-binding protein, and thyroxine-binding prealbumin; these should be monitored on at least a weekly basis as it has been shown that they predict morbidity and mortality in patients with ECFs. Enteral feeding, if it can be successfully provided, at least in part, appears to have a salutary effect on hepatic, intestinal, and respiratory integrity. This is true even if most of the direct nutritional support is given parenterally, but the presence of a significant enteral component is certainly beneficial.
The ability to optimize nutritional status in patients with ECFs depends on successful control and, when possible, elimination of sepsis. The leading cause of mortality in contemporary series is sepsis and multiorgan system failure in the patients with ECFs. Fistula formation is only one of several possible outcomes of bowel perforation or anastomotic breakdown. Enteric contents escaping the bowel lumen may be contained in the local abscess or may spill freely throughout the peritoneal or thoracic cavities, or may track through the abdominal wall and form an enterocutaneous fistula. Closed-space sepsis, such as an abdominal abscess, must be drained before the patient will be able to establish positive nitrogen balance. The liberal, early (emphasize early, not repeatedly) use of computerized tomography, ultrasound, or magnetic resonance imaging will allow early identification and percutaneous management of intra-abdominal abscesses. Peritonitis resulting from free circulation of enteric contents requires urgent laparotomy to contain the spillage and prevent further soilage. If all else fails, a proximal diverting enterostomy may be necessary to control local wound sepsis, which is also treated with antibiotics and wound care techniques employed to protect the surrounding abdominal wall.
Intra-abdominal sepsis is not the only cause of catabolic fever. Blood, urine, and sputum cultures should be obtained to evaluate concomitant infections. In considering antibiotic use in patients with ECFs, one must be very judicious in a choice of antibiotics to avoid selection of multi-agent-resistant organisms, and therefore antibiotics should only be used to treat identified infection using the narrowest spectrum agent available for the shortest duration of time. In the classic study written by Soeters in the 1970s, the average number of antibiotics used throughout such a course of a patient with an ECF was eight or nine, emphasizing the need to think very carefully as to whether antibiotics are needed, and if so, what spectrum should be used. In my view, it is better to tolerate a low-grade fever if the patient is not ill rather than use antibiotics which may be needed later in the course. Finally, it is important to remember that when an abscess is drained, a bacteremia occurs and therefore one should refrain from placing a central line for 12 hours following the drainage of an abscess to avoid contamination of a new catheter.
Management
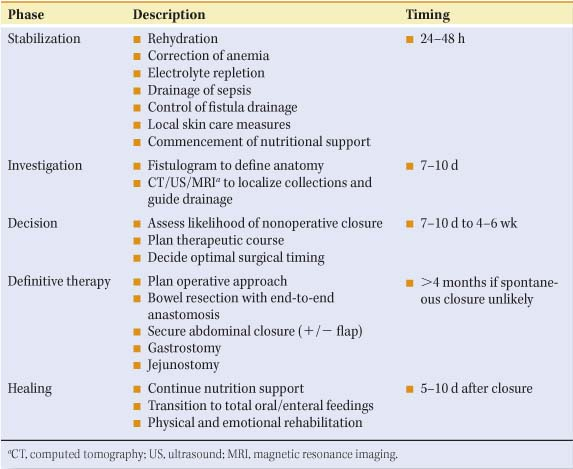
The goal of treating enterocutaneous fistulas is to restore bowel continuity, to achieve enteral nutrition, and to close the fistula. Whether achieved with or without surgical intervention, management of this complex patient is probably best conceptualized in stages with specific goals for each stage (Table 2). One of my mentors, Dr. Francis D. Moore, long-time Chief of the Peter Bent Brigham (at that time), was a master at dividing complex problems into more easily manageable phases.
Stabilization of the patient with identification of the fistula, initial resuscitation, the steps taken to contain fistula drainage, and the beginning of resuscitation take place within the first 24 to 48 hours. Obvious abscesses should be drained, the extracellular fluid space minimized, and the intravascular space resuscitated and expanded to normal levels. Electrolyte disturbances should be corrected, hypoalbuminemia treated,
and severe anemia corrected by the judicious use of packed cells. If sepsis is present, appropriate antibiotics should be started. Though these important steps should take place within the first 24 to 48 hours, they may not be completed for up to 5 days, at which point the patient should be stable and not overtly septic. Some time between 7 and 10 days, if the patient can tolerate it, the surgeon and a senior radiologist should carry out fistulograms to see whether operative intervention will finally be required. The decision as to definitive therapy is considerably later and usually takes place 5 to 6 weeks after the initial discovery. If the decision is made that operative therapy will be required, it is purely elective unless the patient is septic, in which case immediate operation is necessary and it is for patient salvage rather than definitive therapy. If the surgeon is not pushed by sepsis to do this definitive therapy, he/she should wait until the obliterative peritonitis subsides, usually at 4 to 5 months with a patient who has been totally restored with the exception of the fistula, and taken to the operating room. Prior to this, the patient must be sustained with appropriate nutritional support, enteral or parenteral or both, and have physical and emotional rehabilitation during the healing phase which is a continuation of that which takes place prior to operation. Following successful operation, continued rehabilitation, nutritional counseling, and emotional support are necessary to return the patient to his/her original level of function.
and severe anemia corrected by the judicious use of packed cells. If sepsis is present, appropriate antibiotics should be started. Though these important steps should take place within the first 24 to 48 hours, they may not be completed for up to 5 days, at which point the patient should be stable and not overtly septic. Some time between 7 and 10 days, if the patient can tolerate it, the surgeon and a senior radiologist should carry out fistulograms to see whether operative intervention will finally be required. The decision as to definitive therapy is considerably later and usually takes place 5 to 6 weeks after the initial discovery. If the decision is made that operative therapy will be required, it is purely elective unless the patient is septic, in which case immediate operation is necessary and it is for patient salvage rather than definitive therapy. If the surgeon is not pushed by sepsis to do this definitive therapy, he/she should wait until the obliterative peritonitis subsides, usually at 4 to 5 months with a patient who has been totally restored with the exception of the fistula, and taken to the operating room. Prior to this, the patient must be sustained with appropriate nutritional support, enteral or parenteral or both, and have physical and emotional rehabilitation during the healing phase which is a continuation of that which takes place prior to operation. Following successful operation, continued rehabilitation, nutritional counseling, and emotional support are necessary to return the patient to his/her original level of function.
Table 2 Phases of Management for Enterocutaneous Fistulae | |
|---|---|
|
Phase I: Stabilization
By the time a postoperative fistula has become apparent, the patient already has significant deficits in lean body mass, intravascular volume, red cell mass, various serum electrolyte, and serum proteins such as serum albumin and acute phase reactants. Resuscitation should begin with protein-containing solutions such as 5% Albumisol, provided there is no sepsis-related capillary leak, as concentrated salt-poor albumin is too expensive. One could begin with some crystalloid; however, one must be very careful not to overwater the patient and make the patient edematous. It is helpful, as stated earlier, to measure the fistula drainage to get some idea as to what electrolytes are being lost and use these values as a guide to replacement. Electrolyte values should be monitored as replacement continues until stable values are achieved. Red cell mass should be increased to optimize oxygen delivery. Though the current thinking is that a hematocrit of 21% is adequate, I believe this is too low for the patients who are critically ill and opt for a value of a hematocrit of 30 for oxygen delivery. Although some may be happy with a serum albumin in the in the range of 2.5, I believe we are better off with an albumin of 3.0 to 3.3 at the very least in order to make certain that there is not interstitial edema, that the edema does not interfere with wound healing, and that nutrients are absorbed and get to their destination. Albumin should be administered unless there is sepsis-induced capillary leak. Invasive monitoring and central venous or pulmonary artery catheters may be necessary to guide therapy in the patients with poor cardiopulmonary reserve.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree