(1)
Division of Gastroenterology, Department of Medicine, University of Alberta, Edmonton, AB, Canada
Nausea and Vomiting
Differential Diagnosis
NEUROLOGIC
organic—infections, tumors, multiple sclerosis, vestibular nerve or brain stem lesions
drugs—chemotherapy, SSRI, opioids, antibiotics, hormonal therapy, chronic marijuana use
psychiatric—anorexia nervosa, bulimia nervosa, rumination
GASTROINTESTINAL
infections—acute gastroenteritis, food poisoning, UTI, pyelonephritis, pneumonia
neoplastic—gastric, ovarian, paraneoplastic, renal
obstruction—stomach, small bowel, colon, functional, gastric volvulus
postop—vagotomy, gastrectomy, fundoplication
inflammation—esophagus, stomach, duodenum
gastroparesis—ischemic, diabetic, amyloidosis, scleroderma, drugs
others—eosinophilic gastroenteritis, hepatobiliary disease, pancreatic disease, peritoneal irritation, functional gastrointestinal disorders, retroperitoneal fibrosis
METABOLIC
endocrine—diabetes, adrenal insufficiency, hypercalcemia, hyperthyroidism, hyperparathyroidism, hyperemesis gravidarum, porphyria
others—uremia, pregnancy, migraine
IDIOPATHIC
Pathophysiology
REFLEX PATHWAY
afferent—(1) humoral factors (drugs, toxins, neurotransmitter, peptides) → area postrema in floor of 4th ventricle (chemoreceptor trigger zone) → nucleus tractus solitarius (NTS) in medulla serves as central pattern generator for vomiting; (2) neuronal GI tract stimuli → vagus nerve → NTS; (3) nociceptive stimuli → sympathetic nervous system → brain stem nuclei and the hypothalamus
efferent—NTS → paraventricular nuclei of the hypothalamus and the limbic and cortical regions → gastric electromechanical events are perceived as normal sensations or nausea or discomfort → vagus nerve → gastric and lower esophageal sphincter relaxation, retrograde contraction in proximal small bowel and antrum, abdominal muscle contraction and initial cricopharyngeus contraction followed by relaxation seconds before vomiting
Investigations
BASIC
labs—CBCD, lytes, urea, Cr, glucose, Ca, Mg, PO4, AM cortisol, urinalysis
microbiology—urine C&S
imaging—CXR, AXR
SPECIAL
gastroscopy, gastric emptying study
CT head
Management
SYMPTOM CONTROL
H1 antagonists—dimenhydrinate 25–50 mg PO/PR q4h, diphenhydramine 25–50 mg PO/IV/IM q4h, cyclizine 50 mg PO/IM q4h or 100 mg PR q4h, meclizine 25–50 mg PO daily, promethazine 12.5–25 mg PO/IM q4h or 12.5–25 mg PR daily
D2 antagonists—benzamides (metoclopramide 5–10 mg PO/IV/IM q4h), phenothiazine (prochlorperazine 5–10 mg PO q6–8 h, chlorpromazine 10–25 mg PO q4–6 h), butyrophenones (droperidol 1.25–5 mg IM q4h, haloperidol 0.5–1 mg IV/PO q4h)
5HT3 antagonists—ondansetron 4–8 mg PO/IV q8h, granisetron 2 mg PO or 1 mg IV, dolasetron 100 mg PO/IV daily
M1 antagonists—scopolamine 1.5 mg TD q72h
steroid—dexamethasone 4 mg PO/SC/IV BID–TID
tube feed—NJ tube, G tube
TREAT UNDERLYING CAUSE
Related Topics
Chemotherapy-Induced Nausea and Vomiting (p. 254)
Nausea and Vomiting in the Palliative Setting (p. 448)
Dysphagia
Differential Diagnosis
OROPHARYNGEAL
(upper esophagus and pharynx, or upper esophageal sphincter dysfunction)
neurological—stroke, multiple sclerosis, Parkinson’s, dementia, amyotrophic lateral sclerosis, Guillain–Barre, myasthenia gravis, cerebral palsy, Huntington’s, tardive dyskinesia, brain stem tumors, trauma
myopathic—myotonic dystrophy, dermatomyositis, connective tissue disease, sarcoidosis, paraneoplastic
structural—cricopharyngeal bar, Zenker’s diverticulum, cervical webs, oropharyngeal tumors, osteophytes and skeletal abnormality, congenital abnormality, ill-fitting dentures
infectious—syphilis, Lyme disease, botulism, mucositis
metabolic—Cushing’s, thyrotoxicosis, Wilson’s, amyloidosis, Sjögren’s syndrome
iatrogenic—chemotherapy, neuroleptics, postsurgical, radiation
functional (globus sensation)
ESOPHAGEAL
(body of esophagus, lower esophageal sphincter, cardia)
structural—tumors (benign, malignant), esophagitis/stricture (reflux, caustic/erosive, infectious, eosinophilic, pill, radiation), tylosis, diverticula, iatrogenic (post-surgery, radiation), esophageal ring/web, extrinsic compression (enlarged aorta, left atrium, mediastinal mass, lung cancer, lymphoma, osteophytes, subclavian artery)
motility—achalasia, scleroderma, Chagas disease, diffuse esophageal spasm, hypertensive lower esophageal sphincter, nutcracker esophagus, non-specific esophageal motility disorders
Clinical Features
DIAGNOSTIC CLUES
—history of heartburn may suggest GERD leading to erosive esophagitis, peptic stricture, or esophageal adenocarcinoma. History of atopic diseases especially in a young adult with recurrent dysphagia may suggest eosinophilic esophagitis. Also check for odynophagia, regurgitation, hematemesis, coffee ground emesis, respiratory symptoms, weight loss, and medication history (tetracycline, bisphosphonates, potassium supplements)
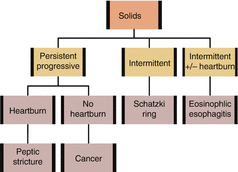
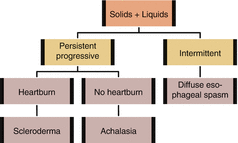
PRACTICAL APPROACH TO DYSPHAGIA
1.
Features of oropharyngeal dysphagia (problems initiating swallowing, extending neck/arms when swallowing, changes in speech, coughing, choking, or nasal regurgitation)? Consider workup for oropharyngeal dysphagia. Otherwise, proceed to step 2
2.
Difficulty swallowing both solids and liquids? If yes, consider motility disorders and proceed to step 3. If progressing from solids to liquids, consider structural disorders and proceed to step 4
3.
For motility disorders, is the dysphagia progressive? If yes, consider achalasia or scleroderma. If intermittent, consider diffuse esophageal spasm or non-specific esophageal motility disorder
4.
For structural disorders, is the dysphagia progressive? If yes, consider tumors and peptic stricture. If intermittent, consider esophageal ring
5.
Any caustic ingestion history?
Investigations
BASIC
imaging—barium swallow (esophageal), videofluoroscopy (oropharyngeal)
swallowing assessment—occupational therapy or speech pathology
SPECIAL
gastroscopy—for esophageal lesions and biopsy for eosinophilic esophagitis
esophageal manometry—definitive for achalasia, useful for diffuse esophageal spasm
p H monitoring—for refractory GERD, especially if gastroscopy normal
fiberoptic nasopharyngeal laryngoscopy—for oropharyngeal dysphagia
Management
SYMPTOM CONTROL
—postural/nutritional/behavioral modifications, swallowing rehabilitation, esophageal dilation
TREAT UNDERLYING CAUSE
Specific Entities
ACHALASIA
pathophysiology—a motor disorder with lack of peristalsis in the body of the esophagus and incomplete relaxation of the lower esophageal sphincter on manometry
diagnosis—endoscopy is essential for ruling out malignancy (“pseudoachalasia”). Barium swallow (beak-like narrowing), esophageal manometry (definitive)
treatments—endoscopic intrasphincteric injection of botulinum toxin, pneumatic dilation, and surgical myotomy
INFECTIOUS ESOPHAGITIS
pathophysiology—common organisms include Candida albicans, CMV, and HSV. Happens more commonly in immunocompromised host
diagnosis—gastroscopy and biopsy/viral cultures
EOSINOPHILIC ESOPHAGITIS
pathophysiology—food allergens and genetic factors leading to eosinophilic infiltration and stricture (frequently presents in young males with esophageal foreign body)
diagnosis—gastroscopy (esophageal trachealization) and biopsy
treatments—control reflux, dilatation, dietary modification, fluticasone (administered as MDI and swallowed), and oral steroids (viscous budesonide slurry)
Related Topics
Esophageal Cancer (p. 215)
Stroke (p. 337)
Dyspepsia
Differential Diagnosis
NON-GASTRIC CAUSES
—cardiac (myocardial infarction), pulmonary (pneumonia), hepatobiliary (biliary colic), pancreatic (pancreatitis), colonic (irritable bowel syndrome), musculoskeletal, dietary indiscretion
PEPTIC ULCER DISEASE
(PUD, 10–20%)—H. pylori, ASA, NSAIDs (COX-2 inhibitors slightly decreased risk), cancer, Zollinger–Ellison, smoking
MEDICATION SIDE EFFECTS
—NSAIDs, ASA, theophylline, calcium channel blockers, erythromycin, metronidazole, bisphosphonates, orlistat, acarbose, iron, potassium supplements
GASTROESOPHAGEAL REFLUX DISEASE
(GERD, 20%)
★ACIDS★
Acid hypersecretion—Zollinger–Ellison disease
Alcohol abuse
Connective tissue disease—scleroderma
Infections of esophagus—CMV, HSV, candidiasis
Diabetic gastroparesis
Drug therapy
Smoking
NON-ULCER DYSPEPSIA
(50%)—cause unclear. Diagnosis of exclusion (rule out organic cause)
Pathophysiology
COMPLICATIONS OF PUD
—perforation, hemorrhage, gastric outlet obstruction, pancreatitis
COMPLICATIONS OF GERD
—esophageal complications include esophagitis, esophageal ulcer, esophageal stricture, and Barrett’s esophagus. Extra-esophageal complications include asthma, aspiration, chronic cough, hoarseness, chronic laryngitis, and dental erosions
Clinical Features
SYMPTOM DEFINITIONS
dyspepsia—chronic or recurrent epigastric pain, often with regurgitation, heartburn, bloating, nausea, and post-prandial fullness (indigestion)
heartburn—retrosternal burning sensation secondary to lower esophageal sphincter relaxation = more specific for GERD
RATIONAL CLINICAL EXAMINATION SERIES: CAN THE CLINICAL HISTORY DISTINGUISH BETWEEN ORGANIC AND FUNCTIONAL DYSPEPSIA?
LR+ | LR– | |
|---|---|---|
Organic dyspepsia | ||
Diagnosis reached by the clinician or computer model | 1.6 | 0.46 |
Peptic ulcer disease | ||
Diagnosis reached by the clinician or computer model | 2.2 | 0.45 |
Esophagitis | ||
Diagnosis reached by the clinician or computer model | 2.4 | 0.5 |
APPROACH—“functional dyspepsia is defined as pain or discomfort centered in the epigastrium with a normal endoscopy. Neither clinical impression nor computer models that incorporated patient demographics, risk factors, history items and symptoms adequately distinguished between organic and functional disease in patients referred for endoscopic evaluation of dyspepsia”
JAMA 2006 295:13
PRACTICAL APPROACH TO DYSPEPSIA
1.
Consider non-gastric causes of dyspepsia (cardiac, pulmonary, hepatobiliary, colonic, musculoskeletal, medications, and dietary indiscretion) and investigate those causes if likely. Otherwise proceed to step 2
2.
If age >50 or alarm symptoms ★Very BAD★ (Vomiting, Bleed/anemia, Abdominal mass/weight loss, Dysphagia), refer for gastroscopy to check for gastric cancer. Otherwise proceed to step 3
3.
If ASA or NSAIDs use, stop medications if possible. If not, consider empiric proton pump inhibitor/H2 blocker trial and proceed to step 4
4.
If GERD predominant symptoms (heartburn, regurgitation), treat as GERD. Otherwise, proceed to step 5
5.
If H. pylori urea breath test positive, treat with triple therapy. Otherwise, proceed to step 6
6.
If none of the above, diagnosis of non-ulcer dyspepsia
Canadian Dyspepsia Working Group. Can J Gastroenterol 2005 19:5
Investigations
BASIC
labs—CBCD, lytes, glucose, AST, ALT, ALP, bilirubin, lipase, Ca, albumin
imaging—upper GI series, US abd, CT abd
SPECIAL
urea breath test
H. pylori serology
24- h esophageal p H monitoring
endoscopy with biopsy—urease test, C&S for H. pylori
proton pump inhibitor test—sens 78% for GERD
Management
PEPTIC ULCER DISEASE
—avoid NSAID use. Antisecretory treatment (ranitidine 150–300 mg PO BID, omeprazole 20–40 mg PO daily, lansoprazole 15–30 mg PO daily, pantoprazole 40 mg PO daily; all 30–60 min before meals; 8 week course). H. pylori eradication (★CAO★: clarithromycin 500 mg PO BID, amoxicillin 1 g PO BID, omeprazole 40 mg PO daily × 10 days; ★CMO★ (if penicillin allergy): clarithromycin 500 mg PO BID, metronidazole 250 mg PO QID, omeprazole 40 mg PO daily × 10 days; ★BMT★ (if macrolide allergy or failed first line): bismuth 30 mL PO QID, metronidazole 250 mg PO QID, tetracycline 500 mg PO QID × 2 weeks)
GERD
—lifestyle changes (avoid coffee, alcohol, chocolate, high-fat meals, acidic or spicy foods. More frequent, smaller portions, exercise/weight loss, smoking cessation, elevate head of bed, loose garments). Antisecretory treatment (proton pump inhibitors more effective than H2 blockers for esophagitis. Use antacids as breakthrough). Nissen fundoplication
NON-ULCER DYSPEPSIA
—lifestyle changes (avoid alcohol, caffeine, tobacco). Antisecretory treatment (see above). H. pylori eradication (may or may not relieve symptoms). Promotility agent (domperidone)
Related Topics
Esophageal Cancer (p. 215)
Gastric Cancer (p. 217)
Gastric Lymphoma (p. 193)
Specific Entities
GERD
causes—obesity, lower esophageal sphincter pressure (transient relaxation of lower esophageal sphincter), decreased esophageal peristalsis, gastric acid hypersecretion, delayed gastric emptying, anatomic disruption lower esophageal sphincter (hiatal hernia)
pathophysiology—reflux of stomach contents, leading to a multitude of symptoms including heartburn, regurgitation, dysphagia, chest pain, complicated by esophagitis, esophageal stricture, Barrett’s esophagus, and esophageal adenocarcinoma
clinical features—esophageal (heartburn, regurgitation), extra-esophageal (wheeze, cough, pneumonia, waterbrash, hoarseness, sore throat, globus, dental erosions)
diagnosis—clinical based on symptoms (≥2/week). Endoscopy to look for complications and rule out other potential diagnoses
NEJM 2008 359:16
NSAIDS-INDUCED GASTROPATHY
pathophysiology—NSAIDs inhibit COX-1 (normally protective effect through mucus secretion, bicarbonate secretion, mucosal circulation) and COX-2 (inducible inflammatory activity, also in kidneys). It also has direct toxic mucosal effect → dose related but even low dose baby ASA may contribute to ulcer formation. Overall ~20% patients on NSAIDs develop ulcers. Risk factors include age >60, pre-existing peptic ulcer, multiple NSAIDs, high-dose NSAIDs, concomitant glucocorticoid or anticoagulant therapy
treatments—primary prophylaxis includes proton pump inhibitor and misoprostol. If ulcer developed while on NSAIDs but must continue, should give proton pump inhibitor
BARRETT’S ESOPHAGUS
pathophysiology—prolonged heartburn → intestinal squamous metaplasia (abnormal salmon-colored mucosa extending proximally from the gastroesophageal junction to the normal pale esophageal mucosa) → dysplasia → adenocarcinoma of esophagus and gastric cardia. Barrett’s develops in 5–8% of patients with GERD. Transformation to low-grade dysplasia 4%/year, high-grade dysplasia 1%/year and cancer 0.5%/year
diagnosis—screen with surveillance endoscopy every 2–3 years if age ≥50, white race, male, obese, chronic GERD, or presence of hiatal hernia. Mucosal biopsy after the initial diagnosis of Barrett’s esophagus to look for dysplasia. Once diagnosed with Barrett’s, endoscopy with biopsy every 3–5 years, 6–12 months if low-grade dysplasia
treatments—high-grade dysplasia should be evaluated for esophagectomy, endoscopic mucosal resection, or ablative therapy
GASTROPARESIS
causes—systemic diseases (diabetes, scleroderma), drugs (anticholinergic agents, narcotics), idiopathic
pathophysiology—impairment of gastric emptying due to dysfunction of the neuromuscular unit → dyspepsia, bloating, nausea, vomiting, and weight loss
diagnosis—gastric emptying study, barium swallow, gastroscopy
treatments—frequent, small, low-fat, low-fiber feedings, prokinetic agents (metoclopramide 10 mg PO TID ac meals, erythromycin 250 mg PO TID ac meals, domperidone 10 mg PO QID), nutritional support
NEJM 2007 356:8
HELICOBACTER PYLORI
pathophysiology—chronic inflammation → causative role in 50–80% of duodenal ulcers, 40–60% of gastric ulcers, 80% of gastric cancers, and 90% of gastric lymphomas
diagnosis—urea breath test (sens 90%, spc 95%. Particularly good in post-treatment setting; testing for eradication should be performed off antibiotic and proton pump inhibitor therapy), serology (sens 90%, spc 80%) is of limited value as it tests for IgG which only indicates previous exposure, endoscopy (culture, histologic assessment, urease testing)
treatments—see H. PYLORI ERADICATION above
Acute Abdominal Pain
Differential Diagnosis
GI
—peptic ulcer disease, pancreatitis, cholangitis, hepatitis, cholecystitis, inflammatory bowel disease, gastroenteritis, appendicitis, diverticulitis, bowel obstruction (small, large), volvulus
GU
—pyelonephritis, renal colic, cystitis, prostatitis, testicular torsion, inguinal hernia
GYNECOLOGIC
—ectopic pregnancy, ruptured ovarian cyst, pelvic inflammatory disease, fibroid torsion, endometriosis, endometritis
VASCULAR
—acute mesenteric ischemia, ischemic colitis, chronic mesenteric ischemia, abdominal aortic aneurysm rupture
SYSTEMIC
—Addison’s disease, diabetic ketoacidosis, uremia, hypercalcemia, porphyria, familial Mediterranean fever
OTHERS
—myocardial infarction, pneumonia, splenic injury, shingles, musculoskeletal, peritonitis
Pathophysiology
CAUSES OF ABDOMINAL PAIN
—any intra-abdominal organs (e.g. GI, GU, gynecological, spleen) × (ischemia, infection, obstruction, tumors) + systemic causes + referred pain
Clinical Features
HISTORY
—characterize abdominal pain (onset, location, duration, severity, radiation, aggravating and relieving factors), N&V, bleeding, fever, inquire about last menstrual period and pregnancy if female, past medical history (CAD, diabetes, hypertension, renal stones), past surgical history (abdominal adhesions), medication history (analgesics)
PHYSICAL
—vitals, respiratory and cardiac examination, abdominal examination, CVA tenderness, pelvic and rectal examination
APPENDICITIS SEQUENCE
—vague pain initially located in the epigastric or periumbilical region; anorexia, nausea, or non-sustained vomiting; migration of the initial pain to the RLQ; low-grade fever
RATIONAL CLINICAL EXAMINATION SERIES: DOES THIS PATIENT HAVE APPENDICITIS?
Sens | Spc | LR+ | LR– | |
|---|---|---|---|---|
History | ||||
Migration of pain to RLQ | 64% | 82% | 3.18 | 0.5 |
RLQ pain | 81% | 53% | 8.0 | 0.15 |
Pain before vomiting | 100% | 64% | 2.76 | – |
No similar pain previously | 81% | 41% | 1.5 | 0.32 |
Physical | ||||
Rigidity | 27% | 83% | 3.76 | 0.82 |
Fever | 67% | 79% | 1.94 | 0.58 |
Rebound tenderness | 63% | 69% | 3.7 | 0.4 |
Psoas sign | 16% | 95% | 2.38 | 0.90 |
Obturator sign | – | – | – | – |
Rectal exam | – | – | – | – |
Combination of Findings | ||||
Alvarado score ≥7 | 81% | 74% | 3.1 | 0.26 |
APPROACH—“migration of pain, RLQ pain and pain before vomit suggest appendicitis. Rigidity, positive psoas sign, fever and rebound tenderness increase likelihood of appendicitis. Absence of above and similar pain previously suggest appendicitis is less likely”
JAMA 1996 276:19
UPDATE—The Alvarado score (MANTRELS–Migration, Anorexia-acetone, Nausea-Vomiting, Tenderness RLQ [2 points], Rebound pain, Elevation of temperature, Leukocytosis [2 points], Left shift) is a user-friendly and powerful tool for assessing likelihood of acute appendicitis
The Rational Clinical Examination. McGraw-Hill, 2009
DISTINGUISHING FEATURES BETWEEN PERITONITIS, SMALL BOWEL OBSTRUCTION, AND ABDOMINAL WALL PAIN
peritonitis—rigidity (LR+ 5.1), guarding (LR+ 2.0), rebound tenderness (LR+ 2.0), positive cough test (LR+ 2.0). Other special tests include Rovsing’s sign, psoas sign (flexion of hip against resistance increases abdominal pain), obturator sign (internal rotation of hip increases abdominal pain), and rectal/pelvic examination
small bowel obstruction—visible peristalsis (LR+ 18.8), absent/tinkling/high-pitched bowel sounds (LR+ 5.0), abdominal bloating
abdominal wall pain—Carnett’s test (palpate area of most intense tenderness while patient supine, then palpate again with patient half sitting up. If pain is intra-abdominal, the pain will not increase as tensed rectus muscles protect the underlying viscus)
Related Topic
Acute Pancreatitis (p. 156)
EXAMINATION OF ABDOMINAL MASSES
right upper quadrant mass—liver (downward with inspiration, left lobe, bruit/venous hum), right kidney (downward with inspiration, ballotable, palpable upper border), gallbladder (downward with inspiration, smooth and regular), colon or gastroduodenal (does not move with inspiration, ill-defined mass, high-pitch bowel sounds), lymphoma (does not move with inspiration, usually more central)
left upper quadrant mass—spleen (downward and medially with inspiration, notch, bruit), left kidney (downward with inspiration, ballotable, palpable upper border), colon (splenic flexure), gastric or pancreatic (ill-defined mass, difficult to clearly differentiate these masses on examination), lymphoma (does not move with inspiration, usually more central)
right lower quadrant mass—colon, distal small bowel, or appendix (lower GI masses are ill-defined and difficult to clearly differentiate on examination), ovary, uterus, fallopian tube (pelvic structures require bimanual examination), lymphoma (does not move with inspiration, usually more central)
left lower quadrant mass—colon, distal small bowel (lower GI masses are ill-defined and difficult to clearly differentiate on examination), ovary, uterus, fallopian tube (pelvic structures require bimanual examination), lymphoma (does not move with inspiration, usually more central)
Investigations
BASIC
labs—CBCD, lytes, urea, Cr, glucose, AST, ALT, ALP, bilirubin, lipase, amylase, lactate, INR, PTT, Ca, albumin, urinalysis, urine βhCG (if ♀ of reproductive age)
microbiology—urine C&S, stool C&S
imaging—CXR, AXR, US abd/pelvic
SPECIAL
imaging—IVP, barium contrast, CT abd
ECG—if suspect cardiac involvement
endoscopy
Diagnostic Issues
APPROACH TO ABDOMINAL X-RAYS
free air—pneumoperitoneum suggests perforation. Look for free air under right diaphragm on CXR view or R lateral decubitus view. On supine abd view, look for outline of bowel wall (normally can only see inside of lumen. If outside of bowel wall also seen, free air present)
small bowel—more central location, valvulae closer together, thin and cross completely. Dilated if >3 cm [1.2 in.], multiple air fluid levels suggest small bowel obstruction
large bowel—more peripheral location, colonic haustra wider apart, thick, and cross part way. Normally some air–fluid levels in ascending colon. Dilated if >5 cm [2 in.]. Thumb printing (mural edema) and dilated bowel suggest toxic megacolon. Check for air in bowel wall (pneumatosis intestinalis)
stool in bowel—cannot distinguish from abscess
kidneys—ureter runs along transverse processes. May see calculi along tract. If see kidney outline, suggests pneumoretroperitoneum
psoas—air around psoas suggests perforated retroperitoneal structures (rectum, duodenum). Lack of psoas outline suggests retroperitoneal inflammation (decreased fat)
biliary structures—common bile duct up to 6 mm in size. Check for air in portal vein or common bile duct (bowel infarction)
other structures—liver, spleen, bones
Management
ACUTE
—ABC, O2, IV hydration. NPO, NG if severe N&V/obstruction. Morphine 2.5–5 mg SC q3–4 h PRN and 1–2.5 mg IV q1h PRN. Dimenhydrinate 50 mg IM/IV q4h PRN
TREAT UNDERLYING CAUSE
—early surgical consult if peritonitis. Empiric antibiotics if fever or suspect peritonitis (ceftriaxone 1 g IV q24h plus metronidazole 500 mg IV q8h, or piperacillin-tazobactam 3.375 g IV q6h, or ciprofloxacin 400 mg IV q12h plus metronidazole 500 mg IV q8h, or meropenem 1 g IV q8h)
Specific Entities
GALLSTONE DISEASE SPECTRUM
—asymptomatic (70%), biliary colic (20%, intermittent obstruction), acute cholecystitis (cystic duct obstruction), choledocholithiasis (common bile duct obstruction), ascending cholangitis (stasis and infection of biliary tract; may be secondary to choledocholithiasis; see p. 156 for more details), gallstone pancreatitis (pancreatic duct obstruction), gallstone ileus, gallbladder cancer
ACUTE CHOLECYSTITIS
pathophysiology—abnormalities of bile acid secretion, mucus generation, and gallbladder motility → gallstone formation → migrate to obstruct the cystic duct and even common bile duct/pancreatic duct → gallbladder inflammation and sometimes secondary infection → gallbladder necrosis and gangrene with perforation in severe cases. Risk factors include older age, obesity, fertility, women (i.e. forty, fat, fertile, female), ethnicity (Aboriginal, Hispanic), TPN, and rapid weight loss
RATIONAL CLINICAL EXAMINATION SERIES: DOES THIS PATIENT HAVE ACUTE CHOLECYSTITIS?
HISTORY—RUQ pain, N&V, anorexia, fever
PHYSICAL—Murphy sign (arrest of inspiration while palpating the gallbladder during a deep breath), guarding, rigidity, RUQ mass, rebound, rectal tenderness
Sens | Spc | LR+ | LR– | |
|---|---|---|---|---|
Murphy sign | 65% | 87% | 2.8 | 0.5 |
RUQ tenderness | 77% | 54% | 1.6 | 0.4 |
Bedside US with gallstones and positive sonographic Murphy sign | – | – | 2.7 | 0.13 |
INVESTIGATIONS—leukocytosis, ALP >120 U/L, elevated ALT or AST, elevated bilirubin
APPROACH—“no single clinical finding or laboratory test carries sufficient weight to establish or exclude cholecystitis without further testing (i.e. ultrasound). Clinical gestalt (without ultrasound) is estimated to have LR+ 25–30, bringing the probability of cholecystitis from 5% pretest to 60% post test. The evaluation of patients with abdominal pain suggestive of cholecystitis will continue to rely heavily on clinical gestalt and diagnostic imaging”
JAMA 2003 289:1
UPDATE—clinicians with training and experience in bedside ultrasound can visualize the gallbladder in most patients. For patients with low pretest probability of cholecystitis and definitively negative bedside ultrasound, formal ultrasound may not be required.
The Rational Clinical Examination. McGraw-Hill, 2009
diagnosis—US abd, endoscopic US, ERCP, percutaneous transhepatic cholangiography, MRCP, HIDA/DISIDA cholescintigraphy (acalculous cholecystitis), CT abd
treatments—supportive measures include NPO, IV fluids, pain control (NSAIDs, opioids), antiemetics and antibiotics (ceftriaxone 1 g IV q24h plus metronidazole 500 mg IV q8h, or piperacillin-tazobactam 3.375 g IV q6h, or ciprofloxacin 400 mg IV q12h plus metronidazole 500 mg IV q8h, or meropenem 1 g IV q8h). Cholecystectomy (laparoscopic, open) or percutaneous cholecystostomy to facilitate drainage (if non-operative because of high risk). If biliary pain despite cholecystectomy, consider possibility of a retained common bile duct stone, sphincter of Oddi dysfunction, or functional pain
NEJM 2008 358:26
ACUTE MESENTERIC ISCHEMIA
pathophysiology—embolism in the celiac or superior mesenteric artery from valvular heart disease or atrial fibrillation, thrombosis, or shock (low flow state) → sudden and severe periumbilical pain out of proportion with physical findings, N&V, leukocytosis, ↑ lactate, ileus
diagnosis—high clinical suspicion, CT abd (+ contrast). Angiography gold standard
treatments—IV fluids, immediate surgery, anticoagulation if mesenteric arterial embolism or mesenteric venous thrombosis
ISCHEMIC COLITIS
pathophysiology—low-flow state in the mesentery affecting mainly the “watershed” area of the middle colic and inferior mesenteric arteries → hematochezia, diarrhea, abdominal pain
diagnosis—AXR (“thumbprinting” or edematous haustral folds), CT (focal or segmental bowel wall thickening or intestinal pneumatosis with portal vein gas), colonoscopy, laparoscopy
treatments—supportive (hydration), antibiotics
CHRONIC MESENTERIC ISCHEMIA
pathophysiology—↓ blood flow from atherosclerosis of the proximal mesenteric vessels → intestinal angina with post-prandial abdominal pain → fear of eating, extensive weight loss
diagnosis—CT abdomen/pelvis (initial), mesenteric duplex US (sens 90% for stenosis of >50%), CT angiography
treatments—angioplasty, surgical revascularization, management of vascular risk factors
Upper GI Bleed
NEJM 2008 359:9
Differential Diagnosis
PEPTIC ULCER DISEASE (PUD)
—gastric, duodenum
INFLAMMATION
—esophagitis (CMV, medications), gastritis (acute, chronic), inflammatory bowel disease (Crohn’s)
VARICES
—esophagus, stomach
TUMORS
—esophagus, stomach, duodenum
STRUCTURAL
—Mallory–Weiss tear, Boerhaave’s syndrome, Dieulafoy’s lesion, arteriovenous malformation, aortoduodenal fistula, hemobilia
OTHERS
—epistaxis, hemoptysis
Clinical Features
HISTORY
—volume of hematemesis, melena, and hematochezia, vomiting, past medical history (PUD, H. pylori infection, alcohol-related disorders, liver cirrhosis with varices, renal failure, metastatic cancer, heart disease/HF), medication history (anticoagulants, NSAIDs)
PHYSICAL
—acute bleeding, sinus tachycardia, supine hypotension (SBP <95 mmHg), postural pulse increase >30/min or dizziness, anemia (conjunctival, facial or palmar pallor), cirrhosis (facial telangiectasia, palmar erythema, spider angiomas, gynecomastia, abdominal wall veins, Terry’s nails/leukonychia, peripheral edema). Perform a rectal examination and test for fecal occult blood. Examine vomitus or nasogastric aspirate and test for occult blood
BLACK STOOL THAT MAY MIMIC MELENA
—bismuth subsalicylate, iron, spinach, charcoal
RATIONAL CLINICAL EXAMINATION SERIES: DOES THIS PATIENT HAVE A SEVERE UPPER GASTROINTESTINAL BLEED?
LR+ | LR– | |
|---|---|---|
Clinical Factors Distinguishing UGIB vs. LGIB | ||
Prior history of UGIB | 6.2 | 0.81 |
Age <50 years | 3.5 | 0.80 |
Cirrhosis | 3.1 | 0.97 |
History of melena | 5.1–5.9 | 0.06–0.27 |
Melenic stool on examination | 25 | 0.52 |
Nasogastric lavage with blood or coffee grounds | 9.6 | 0.58 |
Clots in stool | 0.05 | 1.2 |
Serum urea nitrogen:creatinine ratio >30 | 7.5 | 0.53 |
Clinical Factors Determining Need for Urgent Evaluation of UGIB | ||
History of malignancy or cirrhosis | 3.7 | 0.83 |
Cirrhosis | 3.2 | 0.89 |
Syncope | 3.0 | 0.95 |
Pulse rate >100/min | 4.9 | 0.34 |
Nasogastric lavage with red blood | 3.1 | 0.32 |
Hemoglobin level <8 g/dL | 4.5–6.2 | 0.36–0.41 |
Serum urea nitrogen >90 mg/dL | 3.6 | 0.45 |
Blatchford score = 0 | 1.2 | 0.02 |
Blatchford score: determined by blood urea, hemoglobin, systolic blood pressure, pulse > 99 beats/min, presentation with melena, presentation with syncope, hepatic disease, cardiac failure
APPROACH—“tachycardia (pulse rate of >100/min; LR, 4.9), a history of cirrhosis or malignancy (LR+ 3.7), hemoglobin level of less than 8 g/dL (LR+ range, 4.5-6.2), or a nasogastric lavage with red blood (LR+ 3.1) increase the likelihood of severe bleeding. All patients with a UGIB should have a Blatchford score, which does not require a nasogastric lavage, to help assess the severity (Blatchford score = 0; LR- 0.02 for identifying patients requiring urgent evaluation). When negative, prediction rules combining symptoms, signs, and routine laboratory test results almost definitively rule out severe UGIB, thereby identifying at least some patients who can be safely evaluated as an outpatient”
JAMA 2012 307:10
RATIONAL CLINICAL EXAMINATION SERIES: IS THIS PATIENT HYPOVOLEMIC? HYPOVOLEMIA DUE TO ACUTE BLOOD LOSS
Sens | Spc | |
|---|---|---|
For moderate blood loss | ||
Postural pulse increment ≥30/min or severe postural dizzines | 22% | – |
Postural hypotension ≥20 mmHg SBP drop | 9% | 94% |
Supine tachycardia | 0% | 96% |
Supine hypotension | 13% | 97% |
For large blood loss | ||
Postural pulse increment ≥30/min or severe postural dizziness | 97% | 98% |
Supine tachycardia | 12% | 96% |
Supine hypotension | 33% | 97% |
NOTE: postural change is measured first with supine vitals counting pulse for 30 s (after waiting 2 min), then standing vitals (after waiting 1 min)
JAMA 1999 281:11
Related Topic
Shock (p. 108)
HYPOVOLEMIA DUE TO VOMITING, DIARRHEA, DECREASED INTAKE, DIURETICS
Sens | Spc | LR+ | LR– | |
|---|---|---|---|---|
Symptoms | ||||
Postural pulse increment ≥30/min | 43% | 75% | 1.71 | 0.8 |
Postural hypotension ≥20 mmHg | 29% | 81% | 1.5 | 0.9 |
Dry axilla | 50% | 82% | 2.8 | 0.6 |
Dry oral/nasal mucous membrane | 85% | 58% | 2.0 | 0.3 |
Dry tongue | 59% | 73% | 2.1 | 0.6 |
Tongue with furrows | 85% | 58% | 2.0 | 0.3 |
Sunken eyes | 62% | 82% | 3.4 | 0.5 |
Confusion | 57% | 73% | 2.1 | 0.6 |
Upper/lower extremity weakness | 43% | 82% | 2.3 | 0.7 |
Speech not clear or expressive | 56% | 82% | 3.1 | 0.5 |
Capillary refill time > normal | 34% | 95% | 6.9 | 0.7 |
APPROACH—“for patients with suspected acute blood loss, severe postural dizziness (preventing upright vitals measurements) or postural pulse increment are predictive. Postural hypotension has no incremental value. For patients with suspected hypovolemia not due to blood loss, severe postural dizziness, postural pulse increment, or dry axilla can be helpful. Moist mucous membranes and tongue without furrows argue against it. Capillary refill time and poor skin turgor have no proven diagnostic value”
JAMA 1999 281:11
Investigations
BASIC
labs—CBCD, lytes, urea, Cr, type/cross-match, PTT, INR, AST, ALT, ALP, bilirubin, albumin, fecal occult blood
imaging—CXR, AXR
gastroscopy
Prognostic Issues
RISK STRATIFICATION FOR PEPTIC ULCER DISEASE
clinical R ockall scoring—age 60–79 = 1; age ≥80 = 2; heart rate >100 beats/min = 1; systolic BP <100 mmHg = 2; co-existing illnesses (ischemic heart disease, HF, other major illness) = 2; co-existing illnesses (renal failure, hepatic failure, metastatic cancer) = 3
complete Rockall scoring—in addition to clinical Rockall score, add the following based on endoscopic findings: no lesion observed, Mallory–Weiss tear = 0; peptic ulcer, erosive disease, esophagitis = 1; cancer of upper GI tract = 2; clean base ulcer, flat pigmented spot = 0; blood in upper GI tract, active bleeding, visible vessel, clot = 2
interpretation—low risk for bleeding or death = clinical Rockall score 0 or complete Rockall score ≤2
RISK OF ULCER RE-BLEED
high – risk features—active spurting/oozing during endoscopy (90% chance), non-bleeding visible vessel (50% chance), adherent clot (25–30% chance). If none of above factors and clinically not severe bleed, very low chance of rebleed and may consider discharging shortly after. Other factors include size and location of ulcer
low – risk features—flat spot (10% chance), clean ulcer base (3–5% chance)
Management
ACUTE
—ABC, O2, IV hydration (two large-bore IVs). Transfusion (especially if hemoglobin <70 g/L [<7 g/dL], platelets <50 × 109). NPO, consider NG tube. Hold antihypertensive and diuretic therapy. If prolonged PT/PTT, vitamin K 10 mg IV (small risk of anaphylaxis) and FFP 2–4 U IV or unactivated prothrombin complex concentrates (PCC) 1000–3000 U IV (dosing based on INR and severity of bleeding), if rapid reversal required. If on heparin, consider protamine infusion (1 mg antagonizes 100 U of heparin—beware of excessive protamine which can cause paradoxical coagulopathy). If suspect varices, octreotide 50 μg IV bolus, then 25–50 μg/h. Pantoprazole 80 mg IV bolus, then 8 mg/h until endoscopy. If cirrhosis and acute variceal hemorrhage, transfuse platelet and FFP PRN, antibiotics for 7 days (ceftriaxone 1 g IV q24h, cefotaxime 1 g IV q8h, ciprofloxacin 400 mg IV q12h, ciprofloxacin 500 mg PO BID, or norfloxacin 400 mg PO BID). Consult GI for gastroscopy and consider erythromycin 250 mg IV 30–90 min before endoscopy for clot lavage
TREAT UNDERLYING CAUSE
—avoid ASA, NSAIDs. Peptic ulcer (endoscopic hemostasis with thermal coagulation/fibrin sealant/endoclips plus 1:10,000 ratio epinephrine injection. After endoscopy, start pantoprazole 80 mg IV bolus if not given already, then 8 mg/h × 72 h [if high-risk lesion], switch to 40 mg PO BID × 1 month then daily). Varices (endoscopy within 12 h with ligation/band/glue/sclerotherapy → balloon tamponade → transjugular intrahepatic portosystemic shunt (TIPS) → portacaval/distal splenorenal shunt, or liver transplant. Continue octreotide for 3 days. Repeat endoscopy every 2–4 weeks until varices obliterated, then at 1–3 months and again every 6–12 months afterward. Consider non-selective β-blocker such as nadolol 40–80 mg PO daily or propranolol 20 mg PO BID. Mallory–Weiss tear (omeprazole 20 mg PO daily). H. pylori eradication (see DYSPEPSIA p. 125 for treatment). Intractable or recurrent bleed (consult surgery. See TREATMENT ISSUES below)
Treatment Issues
CRITERIA FOR SURGICAL CONSULT FOR ULCER BLEED
—hemodynamic instability despite resuscitation (>3 U PRBC), shock, recurrent hemorrhage after two endoscopic attempts, continued slow bleed requiring >3 U PRBC/day), high-risk endoscopic lesion
COMPLICATIONS OF ENDOSCOPY
—perforations, bleeding, sedation-related respiratory failure
DISCHARGE DECISIONS FOR PATIENTS’ PEPTIC ULCER DISEASE
—patients with low-risk of re-bleed (complete Rockall score ≤2, low risk endoscopic features), with Hb >80–100 g/L [>8–10 g/dL] without further need of transfusions, normal INR/PTT, and have adequate social support may be safely discharged home shortly after endoscopy with follow-up, while patients with high-risk features should be admitted and monitored closely
Lower GI Bleed
Differential Diagnosis
UPPER GI SOURCE WITH BRISK BLEEDING
(10%)
INFECTIOUS
—Salmonella, Shigella, Campylobacter, Yersinia, E. coli (EHEC, EIEC), C. difficile, Amoeba
TUMORS
—colorectal cancer, small bowel cancer, polyp
INFLAMMATORY
—inflammatory bowel disease (IBD)
ISCHEMIC
—ischemic colitis
STRUCTURAL
—angiodysplasia, diverticulosis, radiation colitis, hemorrhoids, anal fissure, intussusception, Meckel’s diverticulum
Clinical Features
HISTORY
—volume of bleed, melena, abdominal pain, past medical history (IBD, cancer, diverticulosis), medication history (anticoagulants, antiplatelet drugs, NSAIDs)
PHYSICAL
—acute bleeding, sinus tachycardia, supine hypotension (SBP <95 mmHg), postural pulse increase >30/min or dizziness, anemia (conjunctival, facial or palmar pallor), abdominal tenderness. Perform a rectal examination and test for fecal occult blood
Investigations
BASIC
labs—CBCD, lytes, urea, Cr, type/X-match, PTT, INR, AST, ALT, ALP, bilirubin, albumin
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


