Chapter 15 Gastroenterology
Antacids: Buffers
Pharmacokinetics
 Calcium, magnesium, and aluminum are typically poorly absorbed. However, patients with impaired renal function can have accumulation of these cations.
Calcium, magnesium, and aluminum are typically poorly absorbed. However, patients with impaired renal function can have accumulation of these cations. Divalent and trivalent cations such as Ca2+, Mg2+, and Al3+ may chelate other drugs, interfering with their absorption. Where possible, administration of antacids should be separated from administration of other drugs that require systemic absorption, ideally by 2 hours.
Divalent and trivalent cations such as Ca2+, Mg2+, and Al3+ may chelate other drugs, interfering with their absorption. Where possible, administration of antacids should be separated from administration of other drugs that require systemic absorption, ideally by 2 hours.Side Effects
Ca2+-Containing Buffers
 Hypercalcemia (at high doses): These agents may lead to formation of calculi (milk alkali syndrome). Calculi are solid formations, typically consisting of minerals, which precipitate in organs such as the kidney and obstruct ducts.
Hypercalcemia (at high doses): These agents may lead to formation of calculi (milk alkali syndrome). Calculi are solid formations, typically consisting of minerals, which precipitate in organs such as the kidney and obstruct ducts. Bloating, flatulence, belching, nausea: These effects are caused by the liberation of CO2 from carbonate-containing antacids.
Bloating, flatulence, belching, nausea: These effects are caused by the liberation of CO2 from carbonate-containing antacids.Important Notes
 Although these agents act locally in the stomach, they are not devoid of systemic adverse effects, particularly at higher doses or with chronic use.
Although these agents act locally in the stomach, they are not devoid of systemic adverse effects, particularly at higher doses or with chronic use. As with other antacids, buffers may reduce pH enough to interfere with absorption of drugs that require a low pH for absorption.
As with other antacids, buffers may reduce pH enough to interfere with absorption of drugs that require a low pH for absorption. Al3+ and Mg2+ are often combined into one antacid formulation because of their complementary onset of action (Mg2+ is rapid, Al3+ is slow reacting) and side effects (Mg2+ causes diarrhea, Al3+ constipation).
Al3+ and Mg2+ are often combined into one antacid formulation because of their complementary onset of action (Mg2+ is rapid, Al3+ is slow reacting) and side effects (Mg2+ causes diarrhea, Al3+ constipation). Simethicone is a surfactant added to antacids to decrease bloating. A surfactant is a substance that reduces surface tension, in this case reducing large bubbles into smaller ones. The foaming action of simethicone may also alleviate gastroesophageal reflux.
Simethicone is a surfactant added to antacids to decrease bloating. A surfactant is a substance that reduces surface tension, in this case reducing large bubbles into smaller ones. The foaming action of simethicone may also alleviate gastroesophageal reflux. Sodium bicarbonate, one of the first antacids, is still used in some regions. This formulation has fallen out of favor because of concerns over systemic alkalosis and the impact of sodium on cardiovascular health.
Sodium bicarbonate, one of the first antacids, is still used in some regions. This formulation has fallen out of favor because of concerns over systemic alkalosis and the impact of sodium on cardiovascular health.FYI
 Although the agents in this class have traditionally been referred to as antacids, the term antacid has much wider use and applies to each of the many classes of drugs that reduce acid secretion. The more appropriate term for the agents in this class is buffer, as this describes their mechanism and distinguishes them from other classes.
Although the agents in this class have traditionally been referred to as antacids, the term antacid has much wider use and applies to each of the many classes of drugs that reduce acid secretion. The more appropriate term for the agents in this class is buffer, as this describes their mechanism and distinguishes them from other classes.H2 Antagonists
Moa (Mechanism of Action)
 The amount of gastric acid is largely determined by the secretion of protons (H+) by parietal cells in the stomach, as well as volume of stomach contents.
The amount of gastric acid is largely determined by the secretion of protons (H+) by parietal cells in the stomach, as well as volume of stomach contents. In the parietal cell, the proton pump, H+/K+ ATPase, creates an ion gradient by pumping H+ into the lumen of the stomach. The pump is key to creating the acidic environment of the stomach (pH <1) while maintaining a relatively normal intracellular pH (approximately 7.3).
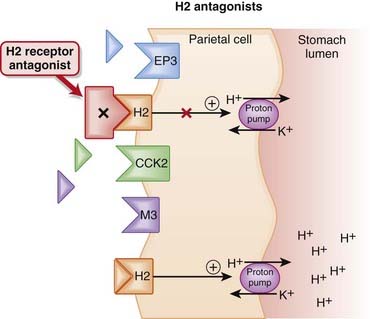
In the parietal cell, the proton pump, H+/K+ ATPase, creates an ion gradient by pumping H+ into the lumen of the stomach. The pump is key to creating the acidic environment of the stomach (pH <1) while maintaining a relatively normal intracellular pH (approximately 7.3). The H2 receptor on parietal cells mediates both the basal and meal-stimulated release of acid. The binding of histamine to the H2 receptor stimulates the proton (H+/K+ ATPase) pump via the second messenger cyclic adenosine monophosphate (cAMP) (Figure 15-1).
The H2 receptor on parietal cells mediates both the basal and meal-stimulated release of acid. The binding of histamine to the H2 receptor stimulates the proton (H+/K+ ATPase) pump via the second messenger cyclic adenosine monophosphate (cAMP) (Figure 15-1). H2 antagonists competitively block the interaction of histamine with H2 receptors, thus reducing stimulation of the proton pump through this receptor.
H2 antagonists competitively block the interaction of histamine with H2 receptors, thus reducing stimulation of the proton pump through this receptor. Nocturnal acid secretion depends largely on histamine; thus the H2 antagonists have a greater impact on nocturnal acid secretion than meal-stimulated acid secretion, which is stimulated by gastrin and acetylcholine (ACh), in addition to histamine.
Nocturnal acid secretion depends largely on histamine; thus the H2 antagonists have a greater impact on nocturnal acid secretion than meal-stimulated acid secretion, which is stimulated by gastrin and acetylcholine (ACh), in addition to histamine. An additional mechanism, by which H2 antagonists are also able to attenuate the gastrin and ACh-stimulated release of gastric acid, has been proposed by some sources. ACh, by binding to muscarinic (M3) receptors, and gastrin, by binding to cholecystokinin (CCK2) receptors, also stimulate the proton pump.
An additional mechanism, by which H2 antagonists are also able to attenuate the gastrin and ACh-stimulated release of gastric acid, has been proposed by some sources. ACh, by binding to muscarinic (M3) receptors, and gastrin, by binding to cholecystokinin (CCK2) receptors, also stimulate the proton pump. It is not clear how H2 antagonism attenuates the ability of gastrin and ACh to stimulate activity of the proton pump, but this would most likely be mediated through second messengers.
It is not clear how H2 antagonism attenuates the ability of gastrin and ACh to stimulate activity of the proton pump, but this would most likely be mediated through second messengers.Pharmacokinetics
 All the H2 antagonists are available in oral formulations. Intravenous and intramuscular formulations of cimetidine, ranitidine, and famotidine are also available.
All the H2 antagonists are available in oral formulations. Intravenous and intramuscular formulations of cimetidine, ranitidine, and famotidine are also available.Side Effects
 Rare central nervous system (CNS) side effects: Confusion, delirium, hallucinations, slurred speech, and headache can occur and are thought to be caused by antagonism of H2 receptors in the CNS. These CNS effects are more likely to occur with intravenous administration or in the elderly.
Rare central nervous system (CNS) side effects: Confusion, delirium, hallucinations, slurred speech, and headache can occur and are thought to be caused by antagonism of H2 receptors in the CNS. These CNS effects are more likely to occur with intravenous administration or in the elderly. Thrombocytopenia (rare): The mechanism has not been established, but theories include bone marrow suppression due to inhibition of DNA synthesis, and the development of platelet antibodies against H2 antagonists.
Thrombocytopenia (rare): The mechanism has not been established, but theories include bone marrow suppression due to inhibition of DNA synthesis, and the development of platelet antibodies against H2 antagonists.Important Notes
 The H2 antagonists are considered to be less effective than the more expensive proton pump inhibitors (PPIs). However, it is important to note that the H2 antagonists are able to reduce daily acid secretion by about 60% to 70%.
The H2 antagonists are considered to be less effective than the more expensive proton pump inhibitors (PPIs). However, it is important to note that the H2 antagonists are able to reduce daily acid secretion by about 60% to 70%. Tolerance can develop to the acid-suppressant effects of the H2 antagonists, occurring as early as 3 days after initiation of therapy. One theory is that secondary hypergastrinemia may stimulate histamine release from enterochromaffin-like cells. Hypergastrinemia is an elevation in gastrin levels in the blood in response to low pH in the stomach. Gastrin stimulates the proton pump to release acid into the stomach.
Tolerance can develop to the acid-suppressant effects of the H2 antagonists, occurring as early as 3 days after initiation of therapy. One theory is that secondary hypergastrinemia may stimulate histamine release from enterochromaffin-like cells. Hypergastrinemia is an elevation in gastrin levels in the blood in response to low pH in the stomach. Gastrin stimulates the proton pump to release acid into the stomach. Cimetidine may also enhance cell-mediated immunity when administered in high doses. This has lead to its use in treating infections such as candidiasis and herpesvirus in immunocompromised patients, as well as warts.
Cimetidine may also enhance cell-mediated immunity when administered in high doses. This has lead to its use in treating infections such as candidiasis and herpesvirus in immunocompromised patients, as well as warts.Evidence
Versus Other Agents for Endoscopy Negative Reflux Disease
 The same 2006 Cochrane review found that PPIs were more efficacious at achieving heartburn remission compared with H2 antagonists (three trials, RR 0.78) and compared with prokinetics (one trial, RR 0.72). Endoscopy-negative reflux disease is simply GERD without any evidence of histologic changes on endoscopic examination.
The same 2006 Cochrane review found that PPIs were more efficacious at achieving heartburn remission compared with H2 antagonists (three trials, RR 0.78) and compared with prokinetics (one trial, RR 0.72). Endoscopy-negative reflux disease is simply GERD without any evidence of histologic changes on endoscopic examination.Proton Pump Inhibitors (PPIs)
Moa (Mechanism of Action)
 The amount of gastric acid is largely determined by the secretion of protons (H+) by parietal cells in the stomach, as well as the volume of stomach contents.
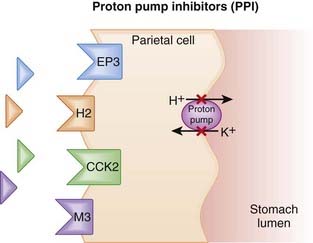
The amount of gastric acid is largely determined by the secretion of protons (H+) by parietal cells in the stomach, as well as the volume of stomach contents. In the parietal cell, the proton pump, H+/K+ ATPase, creates an ion gradient by pumping H+ into the lumen of the stomach. The pump is key to creating the acidic environment of the stomach (pH <1) while maintaining a relatively normal intracellular pH (approximately 7.3) (Figure 15-2).
In the parietal cell, the proton pump, H+/K+ ATPase, creates an ion gradient by pumping H+ into the lumen of the stomach. The pump is key to creating the acidic environment of the stomach (pH <1) while maintaining a relatively normal intracellular pH (approximately 7.3) (Figure 15-2). PPIs enter the parietal cell and bind to the proton pump, resulting in an irreversible inactivation of the pump. This dramatically reduces the amount of H+ that is pumped into the lumen of the stomach, and because the binding is irreversible, the effects of PPIs persist until new pumps are synthesized. The reduction in gastric acid secretion can thus persist up to 48 hours after a single dose.
PPIs enter the parietal cell and bind to the proton pump, resulting in an irreversible inactivation of the pump. This dramatically reduces the amount of H+ that is pumped into the lumen of the stomach, and because the binding is irreversible, the effects of PPIs persist until new pumps are synthesized. The reduction in gastric acid secretion can thus persist up to 48 hours after a single dose. The PPIs tend to be particularly adept at reducing acid secretion because the proton pump is the final and key step in secreting acid (H+) into the lumen of the stomach. All other agents that suppress acid secretion work by reducing gastric H+ concentration act upstream of the proton pump, rather than directly at the proton pump.
The PPIs tend to be particularly adept at reducing acid secretion because the proton pump is the final and key step in secreting acid (H+) into the lumen of the stomach. All other agents that suppress acid secretion work by reducing gastric H+ concentration act upstream of the proton pump, rather than directly at the proton pump. The release of gastrin is modulated by intragastric acid levels, such that higher acidity suppresses gastrin release. Gastrin stimulates ECL cell hyperplasia, which may predispose patients to developing malignancies.
The release of gastrin is modulated by intragastric acid levels, such that higher acidity suppresses gastrin release. Gastrin stimulates ECL cell hyperplasia, which may predispose patients to developing malignancies.Pharmacokinetics
 Although they act on cells in the stomach, PPIs must be absorbed into the systemic circulation from the small intestine. It is from the systemic circulation that they reach the parietal cells of the stomach.
Although they act on cells in the stomach, PPIs must be absorbed into the systemic circulation from the small intestine. It is from the systemic circulation that they reach the parietal cells of the stomach. This is important because the PPIs (ironically) tend to be unstable in an acidic environment. Hence all PPIs have some form of enteric coating, to protect them until they reach the small intestine and can be absorbed. Disruption of this enteric coating (e.g., by splitting the tablet) will likely reduce the bioavailability of the PPI.
This is important because the PPIs (ironically) tend to be unstable in an acidic environment. Hence all PPIs have some form of enteric coating, to protect them until they reach the small intestine and can be absorbed. Disruption of this enteric coating (e.g., by splitting the tablet) will likely reduce the bioavailability of the PPI. All PPIs are prodrugs that are activated in the acidic environment of the parietal cell acid canaliculi. Food intake stimulates acid secretion. Oral PPIs should be taken approximately 30 to 60 minutes before meals. This allows enough time for the PPI to be absorbed into the systemic circulation and to be distributed to the parietal cells. This will also ensure that the PPIs are active at the same time as maximal activation of the proton pumps.
All PPIs are prodrugs that are activated in the acidic environment of the parietal cell acid canaliculi. Food intake stimulates acid secretion. Oral PPIs should be taken approximately 30 to 60 minutes before meals. This allows enough time for the PPI to be absorbed into the systemic circulation and to be distributed to the parietal cells. This will also ensure that the PPIs are active at the same time as maximal activation of the proton pumps.Side Effects
Less Common
 Hypergastrinemia: Gastrin levels become elevated because of the body’s response to chronic gastric acid suppression. This may lead to rebound hypersecretion of gastric acid if the PPI is stopped. There is also concern over the chronic effects of hypergastrinemia, including development of gastric tumors.
Hypergastrinemia: Gastrin levels become elevated because of the body’s response to chronic gastric acid suppression. This may lead to rebound hypersecretion of gastric acid if the PPI is stopped. There is also concern over the chronic effects of hypergastrinemia, including development of gastric tumors.Important Notes
 Of all agents used to treat hyperacidity, PPIs are the most effective at reducing daily acid secretion, capable of reducing acid (basal and stimulated) by 80% to 95%. H2 antagonists are able to achieve a 60% to 70% reduction in acid.
Of all agents used to treat hyperacidity, PPIs are the most effective at reducing daily acid secretion, capable of reducing acid (basal and stimulated) by 80% to 95%. H2 antagonists are able to achieve a 60% to 70% reduction in acid. PPIs inhibit only active proton pumps. Not all proton pumps are active at the same time; therefore, although pumps are irreversibly inhibited once bound by the PPI, it takes a few days to achieve the inhibition of proton pumps seen at steady state.
PPIs inhibit only active proton pumps. Not all proton pumps are active at the same time; therefore, although pumps are irreversibly inhibited once bound by the PPI, it takes a few days to achieve the inhibition of proton pumps seen at steady state. PPIs are often prescribed in combination with other GI drugs and antibiotics for eradication of H. pylori. By increasing intragastric pH, PPIs appear to enhance the antimicrobial activity of these agents. PPIs may also have a minor antimicrobial effect. Some of the more common combinations are listed in Table 15-1.
PPIs are often prescribed in combination with other GI drugs and antibiotics for eradication of H. pylori. By increasing intragastric pH, PPIs appear to enhance the antimicrobial activity of these agents. PPIs may also have a minor antimicrobial effect. Some of the more common combinations are listed in Table 15-1.TABLE 15-1 Combination Therapy for H. pylori Eradication
| Proton Pump Inhibitors | Other Agents | |
|---|---|---|
| Lansoprazole | Clarithromycin | Amoxicillin |
| Omeprazole | Clarithromycin | Metronidazole |
| Pantoprazole | Metronidazole | |
| Rabeprazole | Bismuth subsalicylate | Tetracycline |
Evidence
Versus Other Agents for Endoscopy Negative Reflux Disease
 The same 2006 Cochrane review found that PPIs were more efficacious at achieving heartburn remission compared with H2 antagonists (three trials, RR 0.78) and compared with prokinetics (one trial, RR 0.72). Endoscopy-negative reflux disease is simply GERD without any evidence of histologic changes on endoscopic examination.
The same 2006 Cochrane review found that PPIs were more efficacious at achieving heartburn remission compared with H2 antagonists (three trials, RR 0.78) and compared with prokinetics (one trial, RR 0.72). Endoscopy-negative reflux disease is simply GERD without any evidence of histologic changes on endoscopic examination.Versus H2 Antagonists for Acute Bleeding from Peptic Ulcer
 A 2006 Cochrane review (24 studies, N = 4373 patients) found no difference in mortality between PPIs and controls but did find that PPIs reduced rebleeding (incidence of 10.6% for PPI versus 17.3% control) and surgery (6.1% versus 9.3%, respectively) versus control. No benefit was seen for PPIs versus H2 antagonists with regard to surgery.
A 2006 Cochrane review (24 studies, N = 4373 patients) found no difference in mortality between PPIs and controls but did find that PPIs reduced rebleeding (incidence of 10.6% for PPI versus 17.3% control) and surgery (6.1% versus 9.3%, respectively) versus control. No benefit was seen for PPIs versus H2 antagonists with regard to surgery.Gastrointestinal Cytoprotectants
Moa (Mechanism of Action)
Prostaglandin Analogue
 Protection of the mucosal lining of the stomach can be achieved in two ways: by increasing gastric pH or by enhancing the mucosal barrier that protects the stomach.
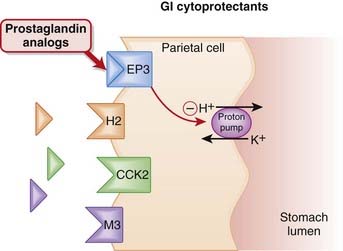
Protection of the mucosal lining of the stomach can be achieved in two ways: by increasing gastric pH or by enhancing the mucosal barrier that protects the stomach. The prostaglandin E receptor 3 (EP3) receptors are found on parietal cells of the stomach and, when stimulated, have an inhibitory effect on the proton pump. The proton pump stimulates the release of hydrogen ions (acid) into the lumen of the stomach.
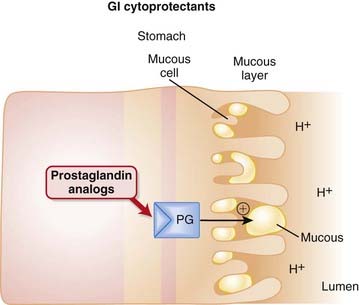
The prostaglandin E receptor 3 (EP3) receptors are found on parietal cells of the stomach and, when stimulated, have an inhibitory effect on the proton pump. The proton pump stimulates the release of hydrogen ions (acid) into the lumen of the stomach. Endogenous PGE2 acts as an agonist at EP3 receptors on parietal cells and reduces activity of the proton pump, thereby reducing secretion of gastric acid (Figure 15-3).
Endogenous PGE2 acts as an agonist at EP3 receptors on parietal cells and reduces activity of the proton pump, thereby reducing secretion of gastric acid (Figure 15-3). PGE2 also contributes to maintenance of the mucosal barrier, stimulating secretion of mucin and bicarbonate and enhancing mucosal blood flow. Mucin is a thick substance that has a protective effect on the lining of the stomach. Bicarbonate helps to raise the pH of the stomach, particularly in the area close to the mucosal lining.
PGE2 also contributes to maintenance of the mucosal barrier, stimulating secretion of mucin and bicarbonate and enhancing mucosal blood flow. Mucin is a thick substance that has a protective effect on the lining of the stomach. Bicarbonate helps to raise the pH of the stomach, particularly in the area close to the mucosal lining. NSAIDs work by inhibiting COX enzymes, thus reducing the amount of PGE2 and subsequently reducing the amount of protective mucus in the stomach. Nonselective (i.e., COX-1 and COX-2) inhibitors are thus implicated in damage to the gastric mucosa.
NSAIDs work by inhibiting COX enzymes, thus reducing the amount of PGE2 and subsequently reducing the amount of protective mucus in the stomach. Nonselective (i.e., COX-1 and COX-2) inhibitors are thus implicated in damage to the gastric mucosa. Therefore a PGE1 analogue such as misoprostol is typically given as an adjunct in patients undergoing NSAID therapy, for the purpose of substituting for the PGE lost with NSAID use (Figure 15-4).
Therefore a PGE1 analogue such as misoprostol is typically given as an adjunct in patients undergoing NSAID therapy, for the purpose of substituting for the PGE lost with NSAID use (Figure 15-4).Sucralfate
 Sucralfate is a complex of sucrose and aluminum hydroxide that forms a viscous paste in aqueous acidic media. This negatively charged paste binds to positively charged proteins in the ulcer, forming a direct protective barrier for up to 6 hours.
Sucralfate is a complex of sucrose and aluminum hydroxide that forms a viscous paste in aqueous acidic media. This negatively charged paste binds to positively charged proteins in the ulcer, forming a direct protective barrier for up to 6 hours. Sucralfate works by multiple mechanisms, both direct and indirect, to enhance the amount of mucus to protect cells lining the stomach.
Sucralfate works by multiple mechanisms, both direct and indirect, to enhance the amount of mucus to protect cells lining the stomach.