Objectives
- Understand how the motility functions of the stomach contribute to the integrated response to a meal
- Describe the muscle layers and their connections to the enteric nervous system that subserve gastric motility
- Define the motility patterns that characterize movement of the stomach under fed and fasted conditions and their control mechanisms
- Describe receptive relaxation and mixing/grinding patterns of motility and their regulation
- Understand how the stomach is emptied, and how this is coordinated with the function of downstream segments
- Describe receptive relaxation and mixing/grinding patterns of motility and their regulation
- Understand the pathophysiology of disease states where gastric motility is abnormal, including gastroparesis, pyloric stenosis, and vomiting
Basic Principles of Gastric Motility
 As we have learned from previous chapters, the stomach is a segment of the gastrointestinal tract in which important aspects of digestion and secretory function are initiated. However, in addition to these functions, which are largely dependent on gastric secretory function, the stomach also plays critical roles that depend on its motility properties.
As we have learned from previous chapters, the stomach is a segment of the gastrointestinal tract in which important aspects of digestion and secretory function are initiated. However, in addition to these functions, which are largely dependent on gastric secretory function, the stomach also plays critical roles that depend on its motility properties.
First, the stomach can be considered as a homogenizer, mechanically breaking down ingested food into an emulsion of small particles with a vastly increased total surface area, thereby amplifying the effects of digestion. Second, the stomach is a critical contributor to the matching of food delivery to the digestive and absorptive capacity of more distal segments of the gut. Under normal circumstances, the stomach allows the delivery of approximately 200 kcal/h into the small intestine, although this may vary somewhat depending on the physical form of the meal (solid vs. liquids) and the nutrient(s) of which it is comprised, as will be described further. The stomach thus serves as a reservoir, allowing food particles to move only slowly into the duodenum to maximize their chances for assimilation. To accomplish this function, the stomach exhibits remarkable pressure/volume characteristics, which are vital in accommodating the volume of the meal without allowing significant reflux of the gastric contents back into the esophagus, or forcing them prematurely into the duodenum. Distention of the stomach also delivers important information to downstream segments of the gastrointestinal tract, as well as contributing to the signaling of satiety. These latter features likely underlie the effectiveness of gastric-reduction surgery for the treatment of morbid obesity, since the small remaining stomach reservoir only allows the patient to take small meals comfortably.
Finally, the stomach possesses distinct motility functions during the fasted state. Most importantly, it has developed mechanisms whereby ingested solids that cannot be digested or mechanically dispersed can be expelled from the stomach under normal conditions. This can be considered a “housekeeping” function, which sweeps undigested materials or ingested foreign objects along the length of the entire gastrointestinal tract, beginning at the stomach. This housekeeping function, mediated by a specific motility pattern known as the migrating motor complex or MMC, accounts for the fact that coins or similar objects that are swallowed by small children will eventually be passed in the feces.
Functional Anatomy of the Gastric Musculature
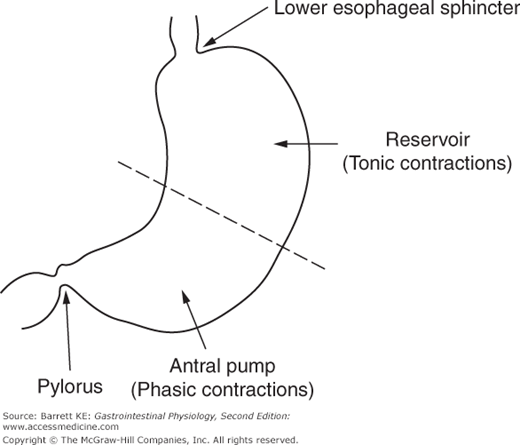
From a motility standpoint, the stomach is a muscular sac with the largest caliber of any intestinal segment. It is a particularly distensible organ, and its ability to increase its volume is also enhanced by the fact that its distension is not highly constrained by adjacent organs, which can be contrasted with the more densely packed organs surrounding more distal segments of the gastrointestinal system. The stomach can also be divided into two functional regions for considerations of motility (Figure 8–1). The proximal stomach, consisting of the cardia, fundus, and proximal portion of the body (corpus) of the stomach, serves primarily as a reservoir and to move gastric contents to the distal stomach. Tonic contractions of the proximal stomach are additionally important in gastric emptying. The distal stomach, on the other hand, consisting of the distal portion of the body and the antrum, serves predominantly to grind and triturate the meal. Finally, the pylorus acts as a sphincter that controls the amount and size of food particles that can exit the stomach in the fed state. Conversely, full relaxation of the pylorus is critical during the housekeeping MMC.
As elsewhere in the gastrointestinal tract, the muscle layers of the stomach consist of a circular layer of smooth muscle arranged circumferentially, and closer to the lumen, and a longitudinal layer that is oriented along the length of the organ. However, because the stomach is shaped as a sac rather than a simple tube, these different muscle layers may assume greater or lesser importance in the different functional regions of the stomach, likely also important for specific motility patterns. Thus, circular muscle is prominent throughout the stomach, although it is notable that it is largely electrically isolated from the circular muscle in the small intestine because of the presence of a connective tissue septum at the level of the pylorus. On the other hand, longitudinal muscle is more prominent in the distal stomach, and these muscle fibers are mostly continuous with those of the duodenum. There is also a small region of obliquely oriented muscle fibers in the lesser curvature of the stomach that is continuous with the gastroesophageal junction, and restricted to the cardia. Finally, the pylorus represents a specialized region of circular muscle at the point where the caliber of the gastric lumen is sharply reduced prior to entry into the duodenum; it serves as a mechanical barrier to food exit that is also enhanced by a folded, redundant mucosa.
The smooth muscle cells of the different functional regions of the stomach also display distinctive contractile properties, which are intrinsic to these cells (myogenic properties) and independent of either neural or humoral input. Most important for our discussion is the distinction between phasic and tonic contractions. Some smooth muscles contract and then relax in a matter of seconds, known as phasic contractions, which are prominent in the distal stomach. Tonic contractions, on the other hand, are sustained contractions that are prominent in the proximal stomach, and may persist for many minutes. Each type of contraction is important in mediating the specific motility properties that are needed for the function of each region of the stomach.
The stomach is richly endowed with both intrinsic and extrinsic neural inputs. The major extrinsic pathways for regulation of gastric motility are parasympathetic in nature, and are contained within the vagus nerve. Most vagal efferents that terminate in the stomach are stimulatory, cholinergic nerves although a few nerves with high thresholds for activation are inhibitory, releasing vasoactive intestinal polypeptide (VIP) and nitric oxide as their major neurotransmitters. Vagal afferents are also critical for the control of motility functions. These are of both mechano- and chemosensitive types, and activate sites in the nucleus tractus solitarius of the dorsal motor nucleus in the brain. In a more limited fashion, sympathetic innervation arrives at the stomach by way of the splanchnic nerve, and these nerves release noradrenaline as a postganglionic inhibitory neurotransmitter at the level of enteric ganglia. The physiological role of sympathetic innervation to the stomach in producing the motility patterns that characterize the response to a meal is minor compared to vagal influences. On the other hand, sympathetic influences may contribute importantly to a decrease in gastric motility during times of threat.
Intrinsic innervation via the enteric nervous system is also critically important to the full expression of gastric motility responses. Indeed, many of the stereotypical motility responses of the stomach are largely, if not wholly, independent of central input. Myenteric neurons of the stomach also provide for coordination of gastric motility functions with those of the more distal segments of the gut, particularly during fasting periods. These nerves also communicate with the pacemaker cells of the intestine, known as interstitial cells of Cajal, located within the circular muscle layers of the stomach and proximal gut. This communication establishes the rate at which contractions of the tissue can maximally occur if an additional excitatory signal is also received, which is known as the basal electrical rhythm, or BER.
Features of Gastric Motility
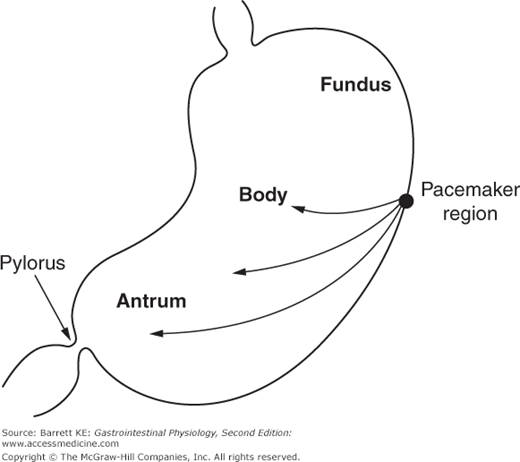
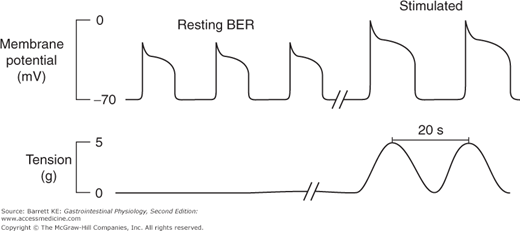
The BER refers to waves of rhythmic depolarization of intestinal smooth muscle cells, which originate at a specific point and then are propagated along the length of the gastrointestinal tract. The pacemaker potentials originating at this point determine the contractile parameters of the stomach as a whole—namely the maximal frequency of contractions, their propagation velocity, and the direction in which they propagate. For the stomach specifically, the waves appear to begin at a point in the body along the greater curvature of the stomach, and then sweep across the stomach toward the pylorus (Figure 8–2). It should also be emphasized that the BER represents only the maximal rate of contraction of the stomach or indeed of any other segment of the gastrointestinal tract. The waves of depolarization that occur in response to the pacemaker activity of the network of interstitial cells of Cajal are not of sufficient magnitude to initiate action potentials in the smooth muscle. Rather, it is only when the release of stimulatory neurotransmitters from enteric nerve endings is superimposed on these waves of depolarization that the threshold for contraction of the smooth muscle will be reached (Figure 8–3). The BER differs in the various intestinal segments. For example, in the stomach the BER is approximately 3 cycles per minute (cpm), whereas the duodenum has a BER of 12 cpm. This presumably reflects the presence of dominant and separate pacemakers in each distinct segment, which then relay electrical information throughout the segment that they control via the corresponding network of interstitial cells of Cajal.
 The ability of the stomach to relax as its volume increases is essential to its reservoir function. The process by which this occurs is referred to as receptive relaxation, and results in a drop in gastric pressure immediately after eating that persists until all solids have been emptied from the stomach. Some further divide this response into two phases—true receptive relaxation, which is a response that occurs coincident with swallowing, and accommodation, a relaxation of the stomach that is mediated by gastric mechanoreceptors that are activated as the wall of the stomach is stretched by the entry of the meal. For our purposes, however, the overall process can be considered as a single, integrated response, which involves vagal input coincident with food intake, vago-vagal reflexes, and intrinsic reflexes mediated wholly within the wall of the stomach (Figure 8–4). Vago-vagal and intrinsic reflexes are triggered by the activation of mechanosensitive nerve endings within the stomach wall. In turn, acetylcholine (ACh) released by vagal pathways acts presynaptically to release additional neurotransmitters that actively relax the gastric smooth muscle layers, particularly in the proximal part of the stomach. Both VIP and nitric oxide have been implicated in this response, although other mediators may also be involved.
The ability of the stomach to relax as its volume increases is essential to its reservoir function. The process by which this occurs is referred to as receptive relaxation, and results in a drop in gastric pressure immediately after eating that persists until all solids have been emptied from the stomach. Some further divide this response into two phases—true receptive relaxation, which is a response that occurs coincident with swallowing, and accommodation, a relaxation of the stomach that is mediated by gastric mechanoreceptors that are activated as the wall of the stomach is stretched by the entry of the meal. For our purposes, however, the overall process can be considered as a single, integrated response, which involves vagal input coincident with food intake, vago-vagal reflexes, and intrinsic reflexes mediated wholly within the wall of the stomach (Figure 8–4). Vago-vagal and intrinsic reflexes are triggered by the activation of mechanosensitive nerve endings within the stomach wall. In turn, acetylcholine (ACh) released by vagal pathways acts presynaptically to release additional neurotransmitters that actively relax the gastric smooth muscle layers, particularly in the proximal part of the stomach. Both VIP and nitric oxide have been implicated in this response, although other mediators may also be involved.