Fungus
Pulmonary fungal infections may be endemic or opportunistic. The truly pathogenic (endemic) fungi may infect healthy as well as immunocompromised hosts, whereas the opportunistic fungi usually infect immunocompromised hosts only. Fungal infections are acquired by inhalation of contaminated soil or, less commonly, through a hematogenous route from other infected foci in the body. Once in the lung, the fungi may elicit either an acute exudative reaction or a granulomatous reaction. Depending on the immunocompetence of the host and the pathogenicity of the fungus, the pulmonary lesions may resolve, progress to chronic inflammation, or disseminate systemically.
Diagnosis of fungal infections depends on demonstration of the fungi by histology and culture. Although fungi are often visible on hematoxylin and eosin (H&E)-stained sections, special stains such as Gomori methenamine silver (GMS) or periodic acid-Schiff (PAS) reagent are often needed for screening and studying the morphologic details. Culture is required for confirmation of diagnosis. Histologically, almost all fungal infections have granulomas with or without a necrotic center, surrounded by lymphocytes, histiocytes, and multinucleated giant cells, with varying degrees of fibrosis in the chronic stage of infection.
Part 1 Aspergillus
Abida K. Haque
The Aspergillus genus has hundreds of species, although three species are most frequently pathogenic: A. fumigatus, A. flavus, and A. niger. Several forms of pulmonary aspergillosis are seen: surface colonization of bronchial mucosa, formation of aspergilloma (fungus ball) in preexisting cavities, allergic aspergillosis in asthmatics, chronic necrotizing aspergillosis in mildly immunocompromised individuals, and invasive pulmonary aspergillosis in severely immunocompromised individuals.
Invasive aspergillosis is the most aggressive form of Aspergillus infection. The early lesions show intense neutrophil response and necrosis, often associated with acute vasculitis, hemorrhage, infarction with formation of targetoid lesions, and cavitation. Occasionally, fungal hyphae are associated with oxalate crystals.
Histologic Features
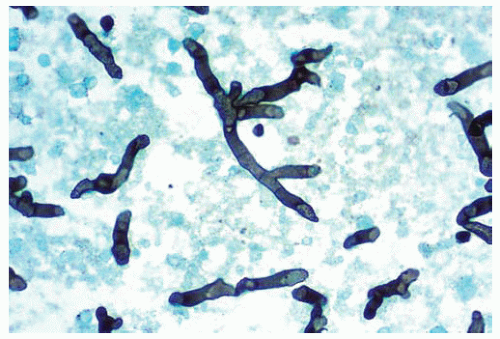
Hyphae are homogeneous, uniform, septate, 3 to 6 μm in width, and have parallel contours, with a dichotomous, acute-angle (45-degree) branching.
The hyphae are basophilic but may appear eosinophilic when degenerated.
Conidial heads are rare, except when the hyphae are exposed to air as in aspergillomas.
Aspergilloma is a fungus ball formed by concentric layers of mycelium within a pre-formed cavity.
The center of the fungus ball has degenerated hyphae that are swollen and show prominent vesicles.
Conidial heads may be seen on the surface and oxalate crystals in the fungus ball.
Necrotizing bronchial aspergillosis is characterized by a pseudomembrane of inflammatory exudates mixed with mucus, inflammatory cells, and abundant hyphae.
The hyphae often invade and destroy the bronchial wall, but no vascular invasion is present.
Invasive pulmonary aspergillosis is characterized by vascular invasion and occlusion with a nodular pulmonary infarction.
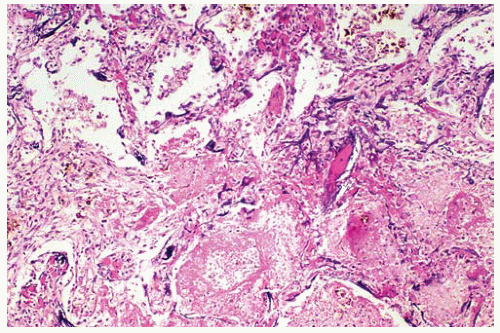
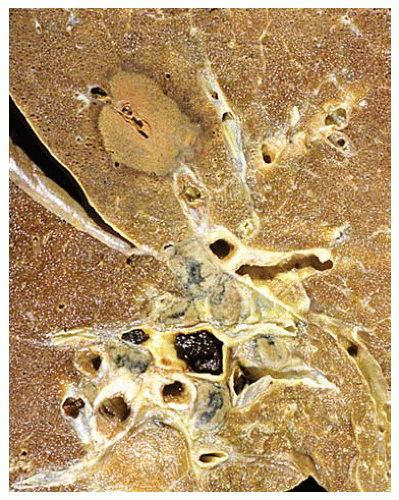 Figure 99.1 Gross figure showing a round area of infarct with a central partially thrombosed vessel characteristic of Aspergillus infarct. |
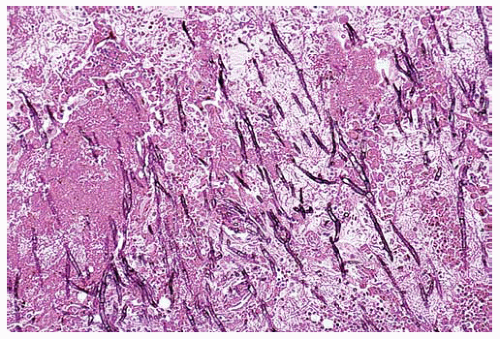 Figure 99.2 Center of the infarct with acutely branching septate hyphae diagnostic of Aspergillus sp. |
Part 2 Mucormycosis
Kirtee Rishi
Mary L. Ostrowski
Abida K. Haque
Mucormycosis is an uncommon infection caused by fungi of the class Zygomycetes, suborder Mucorales. Members of this family include Rhizopus, Absidia, Rhizomucor, Mucor, and Apophysomyces. Rhizopus oryzae is the most frequent agent of the human mucormycosis. It is an uncommon disease that is largely confined to patients with underlying uncontrolled diabetes mellitus, organ transplantation, haematologic malignancy, and neutropenia. These infections are characterized by angioinvasive growth (vasculotropism), resulting in hemorrhage, thrombosis, infarction, and tissue necrosis. Death can result from progressive tissue destruction, uncontrolled hemorrhage, or both.
Histologic Features
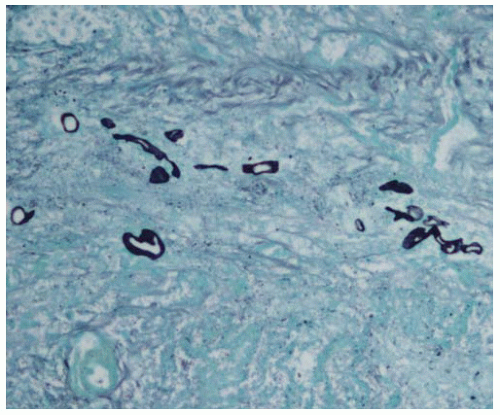
The fungi are broad, irregular, pleomorphic, nonseptate or pauciseptate hyphae, 5 to 20 μm wide.
They show wide-angle and haphazard branching.
The walls of the hyphae are thin, appear basophilic or amphophilic, and stain weakly with GMS and PAS; they are often seen better with H&E stain.
Early lesions show neutrophilic infiltrate and necrosis associated with fungal hyphae, but may be absent in immunocompromised individuals.
Foreign body-type giant cells containing fragments of hyphae, lymphocytes, and histiocytes may be present in patients with subacute infection.
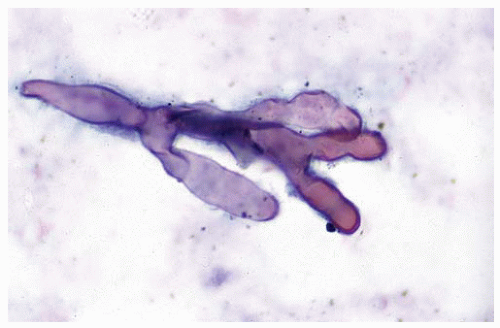 Figure 99.5 Cytology figure showing broad-branching hypha with a pseudoseptation characteristic of Mucor. |
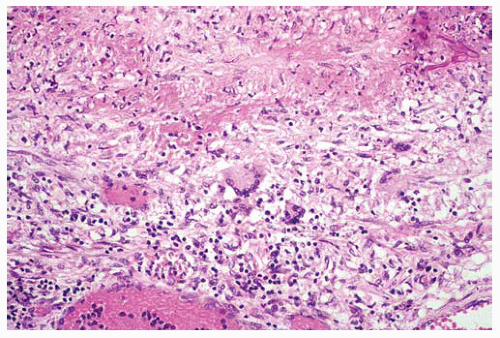 Figure 99.7 The edge of the infarct shows a few centrally located multinucleated giant cells and fungal hyphae in the upper right corner. |
Part 3 Pneumocystis
Abida K. Haque
Carlos Bedrossian
Pneumocystosis is an opportunistic pulmonary infection, caused by Pneumocystis jiroveci (formerly carinii), that occurs almost exclusively in patients with impaired cell-mediated immunity. The most common pulmonary findings are the presence of interstitial infiltrates and characteristic alveolar exudates sometimes progressing to consolidations; rarely nodules, cavities, and pleural effusions are seen. Fatal pulmonary infections may be associated with diffuse alveolar damage (DAD).
Three developmental forms of P. jiroveci may be seen. These include cysts, trophozoites, and sporozoites. Trophozoites attach to alveolar pneumocytes, and mature into precysts and cysts. Mature cysts contain eight haploid sporozoites, that upon release become trophozoites. The developmental forms of P. jiroveci are not clearly seen in routine histologic sections. The trophozoites may be seen with Romanowsky stains (Giemsa, Wright, Diff-Quick), polychrome methylene blue, and Gram stain. The cysts stain with Gomori methenamine silver stain (GMS), toluidine blue, and Gram-Weigert.
With GMS stain, the cysts are seen as thick-walled spherules, 4 to 7 μm, and appear crescent-shaped when collapsed, with a single or paired comma-shaped thickening of the inner cyst wall. The sporozoites are 1 to 2 μm, and trophozoites are 2 to 8 μm in diameter. The trohozoites are amoeboid, with broad pseudopodia and a single nucleus, and often seen in clusters that produce the characteristic foamy exudates.
Cytologic Features
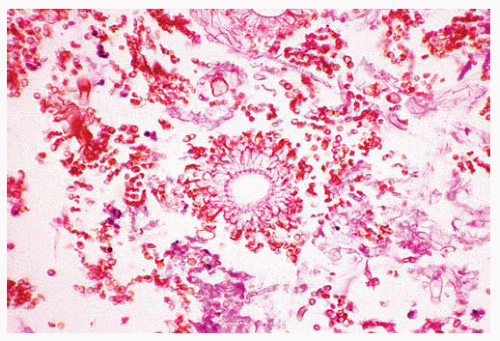
Bronchoalveolar lavage specimens commonly reveal characteristic frothy material, where the outlines of P. jiroveci can be seen within the exudate.
Histologic Features
The characteristic finding is foamy, eosinophilic alveolar exudates, with mild alveolar wall thickening and alveolar pneumocyte hyperplasia.
The alveolar exudate has fine, basophilic dots.
Pneumocystis cysts show internal single or paired distinctive foci of enhanced staining with GMS, which helps to differentiate P. jiroveci from other small fungi. Trophozoites are not seen with GMS stain, and require one of the Romanowsky stains for identification.
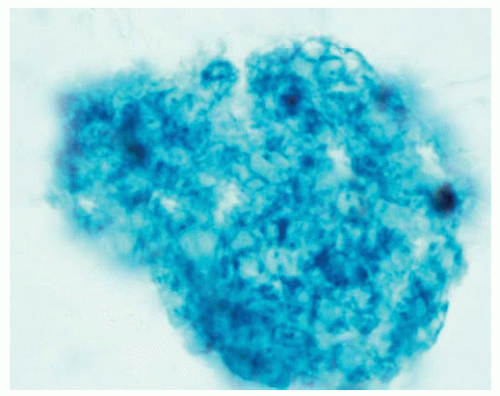 Figure 99.10 Cytology figure of bronchoalveolar lavage specimen showing frothy material characteristic of Pneumocystis.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|