Chapter 1 Fundamentals of chemistry
A knowledge of the materials we are using will help us to understand their structure and properties. This in turn will allow us to use them more effectively and safely. In order to do this we need to apply some fundamental scientific concepts drawn mainly from chemistry.
ORGANIZATION OF MATTER
The main points of the theory are as follow:
Phases
Matter exists in three phases or states:
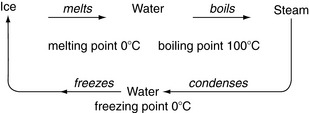
This can be easily explained with reference to water as an example. As a solid it is ice. Ice melts to liquid water. The liquid can then be boiled to form gaseous vapour (steam). Definite temperatures are associated with these changes (Fig. 1.1). Each substance has its own specific temperatures at which these changes of phase take place.
Properties such as boiling points are important as criteria for purity: pure water boils at 100 °C and freezes at 0 °C under the normal ambient atmospheric pressure. If a substance such as salt is added, the boiling point becomes higher and the freezing point becomes lower. This is useful in cold weather: salt put down on steps and roads prevents ice forming until the temperature is very much lower than the freezing point of pure water. We can also separate mixtures of liquids by distillation, which relies on the different boiling points of the components.
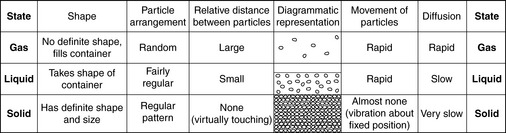
The arrangement and movement of the particles in matter account for its properties in the various phases. In a gas, the particles move freely and at great speed and collide frequently with one another with considerable energy; as a result, a gas completely fills the space available in any container. In a solid, there is no free movement of particles, which occupy fixed positions (although they vibrate around these positions); as a result, solids have definite shape and size. Liquids occupy an intermediate position: the particles are relatively free to move, so that a liquid flows to adopt the shape of its container, but they are attracted to each other sufficiently to keep them together and prevent the particles filling the whole space, as with a gas. The arrangements and motions of particles in the three phases of matter are shown in Figure 1.2. The movement of the particles gives them a property called kinetic energy; the more rapid the motion, the greater the kinetic energy. Heating increases the kinetic energy of molecules, changing them from solids to liquids, and then from liquids to gases.
The process of mixing of gas particles is called diffusion: molecules move from an area of high concentration (such as liquid oil in a dish) to an area of low concentration such as the air in the room. We smell food as it is heated up and cooked due to molecules of gas forming, escaping and diffusing into the air. Diffusion also takes place in liquids as molecules of one substance intermingle and spread out among those of another. Diffusion is important for movement of substances in the body.
Chemical changes
Chemical changes result in the formation of new substances when the composition of the original substance is changed; for example, when a metal reacts with the oxygen of the air it forms a new substance called an oxide (as when iron goes rusty). Essential oils can also react with oxygen, which alters their chemical composition and properties. Other chemical reactions can be initiated by light and are called photochemical reactions (photo means light). The photochemical process of photosynthesis is essential to green plants for the manufacture of food using light as an energy source. Heat will usually speed up chemical reactions, by increasing the kinetic energy of atoms or molecules so that they collide and interact more frequently. Living systems contain complex protein molecules called enzymes which act as catalysts to either speed up or slow down the rates of the chemical reactions occurring in the cells.
Atoms
The structure of the atom is very important and gives the element its properties. An atom is arranged as a central nucleus surrounded by outer electrons. The nucleus is very small but very dense, being responsible for nearly all the mass (weight) of the atom. It is made up of two particles: protons, which carry a positive electric charge (+1) and are given a relative (arbitrary) mass unit of 1; and neutrons, which have no electric charge but have the same relative mass as the proton (a relative mass unit of 1). The nucleus is only 1/100 000 of the diameter of the whole atom.
| Shell 1 | holds up to | 2 electrons |
| Shell 2 | holds up to | 8 electrons |
| Shell 3 | holds up to | 18 electrons |
The individual shells are made up of a number of subshells in which the electrons have different spatial arrangements (the ‘shapes’ of the orbitals differ). The number of subshells available in a given shell is equal to the shell number. So shell 1 comprises only one subshell (its type is designated s). Shell 2 is made up of two subshells (an s type subshell of the same shape as the 1s subshell and a second type designated p type; this is the 2p subshell). Shell 3 comprises three subshells, an s type (3s), a p type (3p), and type designated d, the 3d subshell. An s subshell can hold two electrons, so that shell 1 can hold two electrons in total. A p type subshell can hold six electrons, so shell 2 can hold eight electrons (two in the s subshell and six in the p subshell). The pattern repeats in shell 3, with now the d subshell able to hold 10 electrons. Thus shell 3 can contain two s electrons, six p electrons and ten d electrons, making a maximum of 18 electrons in shell 3. However, in the atoms we will be considering, only the 3s and 3p subshells will be involved as there are not enough electrons to start filling the d shell. Thus, for our purposes, we can consider shell 3 to contain up to 8 electrons.