Chapter 7 Free radicals
How are Free Radicals Formed?
Electrons like to exist in pairs, so when atoms are chemically separated from their covalent bonds (see Chapter 3 ‘Bonds found in biological chemistry’ p. 13) they do not normally split to leave a single unpaired electron. When this does happen, a free radical is formed.
The ‘attacked’ molecule loses its electron and in turn becomes a free radical. This occurs quickly – in much less than a second – and the process rapidly starts a chain reaction that, once started, can cascade and ultimately result in the disruption of a cell.
What Causes Free Radicals?
How do Free Radicals Cause Damage?
Free radicals cause damage in four ways:
Normal Occurrences of Free Radicals
The body needs free radicals for certain functions:
In a healthy individual, the free radicals that are formed by the body are dealt with via a variety of radical ‘quenching’ systems. However, if too many free radicals form then there is a problem.
Reactive Oxygen Species
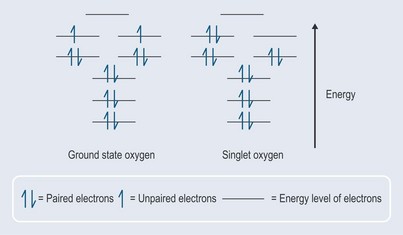
When an oxygen molecule interacts with other molecules to form a covalent bond, it needs to accept two electrons that are spinning in the opposite direction to its own electrons in the outermost orbital or they will not fit (it would be rather like trying to put two like poles of a magnet together, which results in repulsion). In the presence of energy, one of the electrons can change its direction of spin and pairs up with the other one to create singlet oxygen (activated oxygen) (Figure 7.1). This is not as stable as the ground state oxygen and will change back fairly quickly (less than 0.04 microseconds) but in that time it will have affected its surrounding environment.
Singlet oxygen interacts with other molecules in two ways:
Thus, oxygen is activated by two different mechanisms.
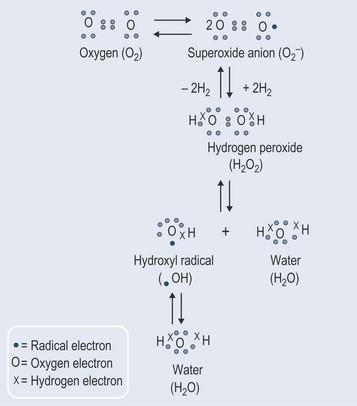
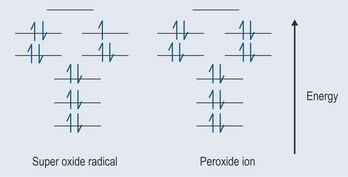
Superoxides
The most important source of the superoxide radical is the electron transport chains in the mitochondria and endoplasmic reticulum (where the cytochrome P450 is stored and works in the liver). The electrons should pass from one component of these chains to another, but the relay system is not perfect and there is thought to be some ‘leakage’.
Effects of Free Radical Damage
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree