Fig. 7.1
Pressure pallor of anterior lividity over weight-bearing points (A). Patterned anterior lividity (B). Tardieu spots and anterior lividity (C).
♦
Extent is minimal with blood loss and is masked by dark pigmentation of the skin
♦
Usually visible within 30 min to 2 h following death, maximal development is usually 8–12 h postmortem; after 8–12 h postmortem, lividity may become fixed and will no longer blanch when pressure is applied to the skin
♦
May form antemortem due to prolonged hypotension
♦
Accelerated rate of fixation with increased temperature and decomposition
♦
Delayed with cool temperature
♦
Different conditions alter the appearance of lividity:
Refrigeration: pink to cherry-red lividity due to retained oxygen
Carbon monoxide: cherry-red lividity due to carboxyhemoglobin
Cyanide: pink to cherry-red lividity due to excessive oxygen retention from inhibition of cytochrome oxidase
Fluoroacetate: cherry-red lividity due to excessive oxygen retention from inhibition of cytochrome oxidase
Hydrogen sulfide: green lividity due to sulfhemoglobin
♦
Advanced livor mortis in which dependent capillaries and venules become overextended, rupture, and coalesce can form pinpoint hemorrhages called Tardieu spots (Fig. 7.1C)
Rigor Mortis
♦
Rigidity of skeletal muscle due to cellular loss of adenosine triphosphate (ATP) and accumulation of lactic acid causing lowering of pH
♦
Rigor mortis begins at death and is usually detectable within 2–4 h following death and may be fully developed 6–12 h following death (Fig. 7.2)


Fig. 7.2
Upper extremities are fixed opposite gravity, indicating movement of deceased from original position after onset of rigor mortis.
♦
The onset of rigor mortis is accelerated in infants and by febrile illnesses, sepsis, increased environmental temperatures, electrocution, seizures, and any excessive muscular activity prior to death
♦
The onset of rigor mortis is reduced or delayed in emaciated and elderly individuals and by cold environmental conditions
♦
Rigor mortis disappears with the progression of decomposition
♦
Can affect involuntary muscles (e.g., causing of pupil disparity postmortem)
♦
Cutis anserina: rigor mortis of arrector pili muscles (i.e., goose flesh)
♦
Cadaveric spasm: rigor mortis in agonal contraction (e.g., clenched fist, articles still held in hand)
Algor Mortis (Body Cooling)
♦
Mechanisms of loss of body heat:
Conduction: heat transferred by direct contact to another object
Radiation: heat transferred to adjacent air by infrared rays
Convection: heat transferred through moving air currents adjacent to the body
♦
Heat loss is decreased by an insulator (e.g., clothing) or increased body fat
♦
Heat loss is increased in cold water
♦
With passage of time, the body temperature will approach the environmental temperature
♦
Prior to death, some conditions may cause an increased body temperature. For example, sepsis, hyperthyroidism, exercise, heat stroke, seizures, drugs (e.g., cocaine, amphetamines, anticholinergics, phencyclidine), and head injuries
♦
Prior to death, some conditions may cause a decreased body temperature. For example, shock, cold environment, and drugs (alcohol, sedatives, opiates, and phenothiazines)
♦
No exact formula is available to calculate the time of death from body temperature; however, in general:
Body temperature decreases 2–2.5 °F/h for the first few hours
Temperature decreases at an average of 1.5–2 °F/h in the first 12 h
In the following 12–18 h, temperature decreases an average of 1 °F/h
Decomposition
♦
Involves two processes:
Autolysis: breakdown of cells and organs by intracellular enzymes, which is accelerated by heat and slowed by cold
Putrefaction: process due to bacteria and fermentation, which is accelerated in patients with sepsis and increased temperature
♦
Decomposition is accelerated by higher environmental temperatures, obesity, heavy clothing, and sepsis
♦
Decomposition is delayed by cold environment and by embalming
♦
Generalized sequence of decomposition:
Decrease of rigor mortis and fixation of livor mortis
Green discoloration in the right lower quadrant of the abdomen
Green discoloration of the head, neck, and shoulders
Swelling of the face due to bacterial gas formation
Marbling, which is caused by hemolysis of blood with a subsequent hemoglobin and hydrogen sulfide reaction, creating green-black discoloration along blood vessels
Generalized bloating and skin slippage and body discoloration from green to black
♦
Additional terminology:
Mummification: dehydration of the body, most prominently seen in dry and hot climates in which skin develops a dry and leathery appearance
Miliaria: white-gray pinpoint discoloration seen below the capsule of the liver, kidney, and spleen and below the endocardium, which is due to the precipitation of calcium and other salts
Adipocere: gray-white and waxy material seen in bodies immersed in water or in damp warm environments in which neutral fats are converted to oleic, palmitic, and stearic acids
Tache noire: brown to black band of discoloration of the bulbar conjunctivae and sclerae resulting from drying
Intrauterine maceration: result of autolysis, not putrefaction
Gastric Emptying
♦
In general, there is variation from day to day in the timing gastric emptying, even in healthy subjects
♦
Larger meals are associated with longer emptying time than smaller meals
♦
Mean emptying half time for liquids is ~1½ h
♦
Mean emptying half time for solids is ~4½ h
♦
Gastric emptying is delayed in individuals with diabetes mellitus, anorexia nervosa, illness, emotional stress, exercise, or severe injury or with the use of some drugs (e.g., alcohols, narcotics, phenothiazines, atropine, and beta-adrenergic drugs)
♦
Gastric emptying times are increased by certain medications (e.g., diazepam) and certain types of exercise
Forensic Entomology and Animal Activity
♦
Different insects are attracted at different stages of decomposition and may aid in the determination of how long a body has been dead
♦
Temperature and humidity are major factors controlling the deposition of eggs and the rate of the development of necrophagous insects
♦
Flies are the most common insects:
Eggs are deposited soon after death in the daytime and take 24–48 h to hatch into maggots
Maggots grow larger to pupa stage (6–10 days); adult flies emerge in 12–18 days
♦
The following factors determine how soon and how many insects appear after death: rate of decomposition, burial, immersion in water, mummification, and geographic location of the body
♦
Following death, household pets such as cats and dogs may feed on the deceased, and the injuries such produced, often involving the face, must be distinguished from antemortem injuries of significance in the cause and manner of death
♦
In addition, postmortem artifacts created by roaches, ants, mice, rats, and other vermin must be differentiated from antemortem injuries
♦
When a death has occurred outdoors, large carnivores (e.g., bears) may feed on the body and cause scattering of remains
Other Methods to Determine Time of Death
♦
Scene markers are unscientific but helpful in determining the time of death:
Useful scene indicators include dates on uncollected mail, date of newspapers in front of the house, a television guide open to a specific date, clothing worn, sales receipts, and interviews with neighbors regarding a decedent’s habits
♦
Analysis of vitreous humor electrolytes:
Vitreous potassium concentration is not reliable for the estimation of time of death
Intracellular potassium is released after death but not at a rate that can reliably be correlated with time since death
Anything that accelerates decomposition increases the release of potassium
Postmortem Chemistry
♦
Analysis of vitreous humor electrolytes can produce characteristic patterns:
Hypertonic dehydration pattern:
•
Increased sodium (>155 mEq/L)
•
Increased chloride (>135 mEq/L)
•
Increased urea nitrogen (VUN) (40–100 mg/dL)
Uremia pattern:
•
Normal to minimal increase in sodium and chloride
•
VUN >150 mg/dL
Low salt pattern (causes include alcoholism, pyloric obstruction, and diuretic treatment):
•
Sodium <130 mEq/L
•
Chloride <105 mEq/L
•
Potassium <15 mEq/L (low relative to decomposition pattern)
Decomposition pattern:
•
Sodium <130 mEq/L
•
Chloride <105 mEq/L
•
Potassium >20 mEq/L
Diabetic pattern:
•
Glucose >200 mg/dL
•
In ketoacidosis, may get acetone
•
Cannot diagnose hypoglycemia postmortem because glucose decreases postmortem
♦
Constituents of blood that are stable postmortem: creatinine, total cholinesterase (can be used to rule out organophosphate poisoning), cortisol, thyroid stimulating hormone (TSH), and calcium
♦
Constituents of blood that exhibit a postmortem increase in concentration: alkaline phosphatase, creatine phosphokinase (CPK), amylase, catecholamines, insulin, magnesium, and potassium
♦
Constituents of blood that exhibit a postmortem decrease in concentration: T4, glucose, sodium, and chloride
Sudden Death from Natural Disease
Classification of Sudden Natural Deaths (Based upon Certainty with Which the Disease Process Identified at Autopsy Represents the True Cause of Death)
♦
Class 1:
Autopsy discloses cause of death with 100% certainty
Accounts for ~5% of natural deaths in medicolegal population
Examples:
•
Myocardial infarct with rupture
•
Dissecting aortic aneurysm with rupture
•
Intracerebral hemorrhage
♦
Class 2:
Autopsy findings are not inconsistent with life, but advanced disease is present that is sufficient to have caused death
Accounts for 90% of natural deaths in medicolegal population
Examples:
•
Advanced heart disease
•
Chronic lung disease
•
Metastatic malignancy
•
Complications of chronic alcoholism
♦
Class 3:
A disease with marginal lethal potential is identified at autopsy, but it is not sufficiently advanced that under ordinary circumstances it would be a competent cause of death
Requires a compelling history and exclusion of other causes to allow for the marginal pathologic findings to be determined to be responsible for the fatality
Infrequent in medicolegal population
Example: witnessed collapse with moderate coronary artery disease present at autopsy and negative toxicology but with no other significant pathologic findings:
•
Despite marginal findings at autopsy, one must conclude that death was due to heart disease
•
Conduction disorders should be considered
♦
Class 4:
The clinical history of a certain disease, which could be lethal, is present; however, lethal structural findings associated with the disease are not readily demonstrable by autopsy
Autopsy is required to exclude an alternative explanation
Example: epilepsy
♦
Class 5:
Cause of death is undetermined after scene investigation, autopsy, and toxicologic studies
There is no evidence that death is due to unnatural causes
Cardiovascular Causes of Death
Atherosclerotic Heart Disease
Clinical
♦
Most common cause of unexpected natural death in the Western world
♦
Signs and symptoms include chest pain, left arm and jaw pain, nausea and vomiting, EKG changes, and elevated cardiac enzymes (e.g., troponin I)
Autopsy
♦
Marked narrowing of coronary arteries, typically >75% of lumen, in which a thrombus may or may not be present
Microscopic
♦
Recent or remote myocardial infarct may be present
♦
May see perivascular and interstitial fibrosis only, and, in some cases, no significant microscopic findings are present
♦
Contraction band necrosis may be present in cases of ischemia but may also be an artifact of resuscitation
Mechanism
♦
Pump failure or sudden arrhythmia
Hypertensive Cardiovascular Disease
Clinical
♦
May or may not have a history of hypertension
♦
No other symptoms, other than sudden cardiac arrest, may exist
Autopsy
♦
Concentric left ventricular hypertrophy
♦
Granular kidneys (i.e., arteriolar nephrosclerosis)
Microscopic
♦
Myocardial fiber hypertrophy
♦
Sclerosis of mural cardiac arteries
♦
Hyaline arteriolosclerosis (hyperplastic arteriolosclerosis in malignant hypertension)
Mechanism
♦
Sudden arrhythmia due to the increased oxygen demand of hypertrophied muscle mass
♦
Hypertensive cardiovascular disease may coexist with atherosclerotic heart disease and lead to the acceleration of coronary atherogenesis
Aortic Stenosis
Clinical
♦
Bicuspid aortic valve (males, 50–70-year-old age group)
♦
Rheumatic valvular disease (women, 35–55 years old; mitral involvement also common)
♦
Degenerative valvular changes involving all three cusps (>60 years of age)
Autopsy
♦
Calcification and obstruction of aortic valve
♦
Left ventricular hypertrophy
Microscopic
♦
Calcification of valve
♦
Myocardial fiber hypertrophy
Mechanism
♦
Sudden arrhythmia due to instability from obstructive blood flow to coronary arteries
Long QT Syndrome (LQTS)
Clinical
♦
Affects approximately 1 in 3,000–5,000 individuals
♦
Individuals with QT interval prolongation greater than 500 ms are highly likely to have LQTS, but in males, greater than 450 ms is significant, and in females, greater than 460 ms is significant
♦
Autosomal dominant and autosomal recessive inheritance patterns are identified, and, in about 15% of cases, the mutation is sporadic
♦
Sixty percent of affected individuals present with syncope, seizures, or sudden death
♦
Forty percent of affected individuals are asymptomatic throughout life
♦
May be the etiology of some cases of sudden infant death syndrome (SIDS)
♦
May suffer drowning or near drowning (in LQT1 most commonly)
♦
Thirteen variants LQT1–LQT13:
LQT1 (KCNQ1, potassium channel gene, chromosome 11) – 25% of cases
LQT2 (KCNH2, potassium channel gene, chromosome 7) – 20–25% of cases
LQT3 (SCN5A, sodium channel gene, chromosome 3) – 5% of cases
LQT4 (ankyrin-B, sodium channel-related gene, chromosome 4)
LQT8 (CACNA1C, calcium channel gene, chromosome 6)
♦
In addition to hereditary forms of LQTS, the condition can also be drug induced, with some commonly prescribed responsible medications including verapamil, erythromycin, fluoroquinolones, haloperidol, thiazides, and methadone
Autopsy
♦
Rule out cardiomyopathy
♦
Must do molecular workup to confirm a channelopathy
Mechanism
♦
Cardiac ion channelopathy leading to lethal dysrhythmia
Hypertrophic Cardiomyopathy
Clinical
♦
Most common cause of sudden death in athletes <35 years of age
♦
Male: female ratio = 2:1
Autopsy
♦
May be symmetrical or asymmetrical thickening of interventricular septum at distal outflow tract, but there is a wide range of phenotypes
♦
Fibrosis of aortic outflow tract corresponding to the anterior leaflet of the mitral valve
Microscopic
♦
Myofiber disarray most prominent in interventricular septum
♦
Plexiform interstitial fibrosis
♦
Thickened intramural coronary arteries
Mechanism
♦
Arrhythmia or cardiac failure
Genetics
♦
Autosomal dominant with variable penetrance, affecting 1 in every 500 people
♦
Mutations in genes that encode sarcomere proteins
♦
Mutation in cardiac myosin-binding protein C gene may result in late onset (middle age and favorable prognosis)
Dilated Cardiomyopathy
Clinical
♦
May be associated with progressive congestive heart failure and early death or with sudden death
♦
May develop secondarily in viral myocarditis, chronic alcoholism, the peri- or postpartum period, hemochromatosis, or with Duchenne’s or myotonic dystrophy
Autopsy
♦
Enlarged flabby heart with all four chambers dilated
♦
Frequent endocardial thickening; may have mural thrombi
Microscopic
♦
Interstitial fibrosis and hypertrophied and attenuated myocytes
Restrictive Cardiomyopathy
Clinical
♦
Associated with systemic diseases such as amyloidosis, hemochromatosis, and glycogen storage diseases
♦
Lower incidence than hypertrophic and dilated cardiomyopathy
♦
Decreased diastolic relaxation and elevated ventricular filling pressure
Autopsy
♦
Myocardium is stiff due to infiltration from a benign or malignant process, scarring, or intracellular accumulations
Microscopic
♦
Dependent on etiology of cardiomyopathy
Myocarditis
Clinical
♦
May be associated with sudden death or progressive heart failure
♦
Most commonly associated with viruses but also bacteria, fungus, protozoa, or autoimmune reactions
♦
May have history of recent viral-like symptoms
Autopsy
♦
Heart may be slightly dilated and flabby
♦
May have focal areas of mottling or may be grossly normal
Microscopic
♦
Diffuse or patchy cellular infiltration, mainly lymphocytes
♦
In some cases, accompanied by marked myocardial fiber necrosis
Mechanism
♦
Ectopic cardiac irritability leading to ventricular dysrhythmias
Myxomatous Mitral Valve
Clinical
♦
Associated clinical condition is mitral valve prolapse, which occurs in ~5–10% of the general population
♦
Primary form has autosomal dominant pattern of inheritance
♦
Midsystolic click heard on auscultation
♦
Most common presenting symptom is palpitations due to premature ventricular contractions (PVCs)
♦
Myxomatous mitral valves are associated with Marfan syndrome, Ehlers-Danlos syndrome, pseudoxanthoma elasticum, osteogenesis imperfecta, straight thoracic spine syndrome, and pectus excavatum
♦
Predisposed to infectious endocarditis and mitral regurgitation
Autopsy
♦
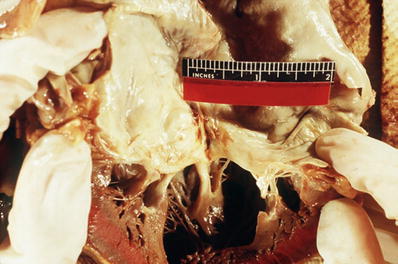
Redundancy of leaflets with prolapse into left atrium (Fig. 7.3), with secondary fibrosis of leaflets eventually occurring
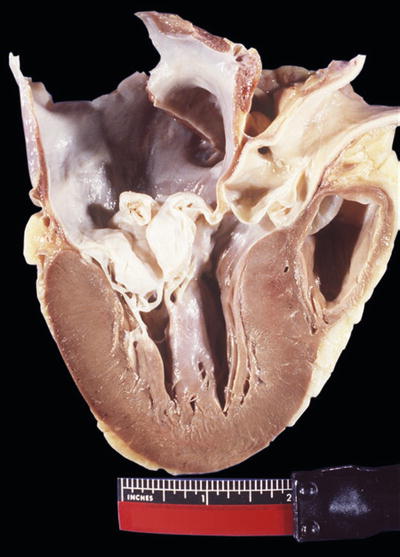

Fig. 7.3
Redundant mitral valve and left ventricular hypertrophy.
♦
Elongation of chordae tendineae
♦
Ventricular friction lesion
Microscopic
♦
Myxomatous degeneration with acid mucopolysaccharides and thickening and mucinous infiltration of zona fibrosa of mitral valve
Mechanism
♦
Frequent PVCs from impact of anterior mitral leaflet on ventricular septum
Aortic Dissection
Clinical
♦
Risk factors include hypertension (coexists in 70–90% of patients) and weakness of the aortic wall (e.g., Marfan syndrome)
♦
Male predominant (3:1)
♦
Increased incidence in pregnancy
♦
Dissection usually heralded by onset of severe sharp chest pain or back pain
Autopsy
♦
Initiating event is a tear in the aortic intima after which blood dissects into the media
♦
Dissection can propagate distally and/or proximally
♦
Most common cause of death is rupture of aortic dissection into either the pericardial space or the left chest cavity
Microscopic
♦
Varies from cystic medial degeneration to no specific pathologic changes
Mechanism
♦
Exsanguination
Pulmonary Thromboembolism
Clinical
♦
Predisposing factors:
Immobility occurring with morbid obesity, postoperative bed rest, pregnancy, or after trauma
Cardiovascular disease (CVD)
Malignant tumors
♦
Hereditary predispositions:
Congenital deficiencies of antithrombin III, protein C, protein S, or plasminogen
Activated protein C resistance (Factor V Leiden)
Hyperhomocysteinemia
Elevated levels of antiphospholipid antibody
♦
Prevalence of venous thromboembolism in antithrombin III, protein C, or protein S deficiency is >50%
♦
Clinical symptoms: sudden death, chest pain, or shortness of breath
Autopsy
♦
Coiled thrombi that do not conform to the shape of the pulmonary arterial tree in which they are found at autopsy, such as postmortem clots would
♦
Less than 95% arise in the large deep veins of lower legs, including popliteal, femoral, and iliac vein
♦
Occasionally, thrombi are also recovered in pelvic veins, especially during pregnancy, or in the periprostatic veins
♦
Rarely, thromboemboli may cross through an interatrial or interventricular defect to the systemic circulation (i.e., paradoxical embolus)
Microscopic
♦
Platelet-fibrin-red blood cell mass with lines of Zahn
♦
Veins may demonstrate organization
♦
May see organizational changes in pulmonary arterial wall if there is survival for several days postembolic event
Mechanism
♦
Occlusion of pulmonary trunk and right ventricle
♦
Minimal flow to left ventricle, leading to sudden death or cardiovascular collapse
♦
Occlusion of smaller arteries may lead to sudden death by vasospasm, sudden increase in blood pressure and right heart failure, or bradycardia due to vasovagal reflex
Cerebrovascular Disease
Intracerebral Hemorrhage (Apoplexy)
Clinical
♦
Associated with hypertension
♦
Most common in middle-aged and elderly
♦
May present with headaches and seizures
Autopsy
♦
Common sites of hemorrhage: external capsule and basal ganglia, cerebellum, thalamus, and pons
Microscopic
♦
Sharp demarcation of hematoma from surrounding brain with death of neurons and glia in adjacent tissue
♦
If survival is >24 h, cerebral edema is present with early organizational changes consisting of monocytes and macrophages invading into edges of hematoma
♦
If preexisting hemorrhage has occurred, hemosiderin may be present, and if small hematoma occurs with survival after several years, residual cysts with brown-orange discolored walls may be present with hemosiderin and gliosis (so-called apoplectic cyst)
Ruptured Berry Aneurysm
Clinical
♦
Accounts for 4–5% of sudden, rapid natural deaths
♦
Median age of patient at the time of rupture is 50 years
♦
Berry aneurysms exist in 1–2% of population
♦
Increased incidence of saccular aneurysms in certain disorders:
Fibromuscular dysplasia
Polycystic kidney disease
Moyamoya disease
Coarctation of the aorta
Autopsy
♦
Large saccular aneurysms at branch points may be easily identified (Fig. 7.5A–C); however, in other cases, aneurysms may be small, extensively damaged when they rupture, and difficult to detect at autopsy
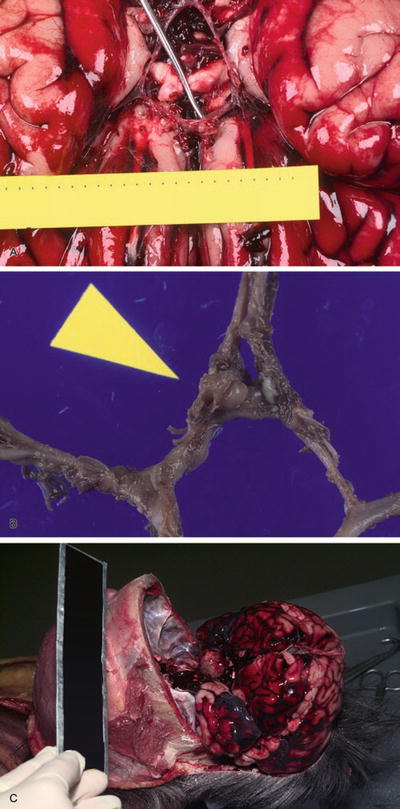

Fig. 7.5
Berry aneurysm (A). Circle of Willis with ruptured berry aneurysm (B). Subarachnoid hemorrhage due to intracranial carotid artery aneurysm (C).
♦
Aneurysms occur at bifurcations of intracranial arteries, with 40% related to intracranial portion of internal carotid artery, usually at juncture of internal carotid and posterior communicating artery, 30% at the anterior communicating artery, 20% at the middle cerebral artery, and 5–10% associated with posterior cerebral arteries and basilar and vertebral artery
♦
Aneurysms may rupture into and dissect into brain parenchyma
♦
When subarachnoid hemorrhage is identified at autopsy, a search for a ruptured berry aneurysm should be performed prior to fixation
Microscopic
♦
Typically thin-walled pouch in which endothelium may be incomplete and with adjacent blood clot
♦
Muscle coat and elastic lamina typically stop abruptly at the neck of aneurysm and wall of aneurysm is fibrotic
Mechanism
♦
Cerebral ischemia with cerebral infarction
Nonvascular Causes of Death
Chronic Alcoholism
Clinical
♦
In binge drinkers, alcohol can be a cardiac irritant and lead to sudden arrhythmia
♦
Long-term alcohol abuse with well-known complications
♦
Alcohol withdrawal with delirium tremens and Wernicke disease
Autopsy
♦
May include a variety of findings:
Alcoholic cardiomyopathy
Acute pancreatitis
Wernicke encephalopathy
Fatty metamorphosis of the liver
Central pontine myelinolysis, which is also seen with rapid correction of hyponatremia
Microscopic
♦
Specific to particular disease processes
Mechanism
♦
Alcohol may function as a cardiac irritant or may cause electrolyte abnormalities (e.g., magnesium deficiency)
Epilepsy
Clinical
♦
The decedent must have an antemortem diagnosis of epilepsy to certify it as a cause of death
♦
An agonal convulsive episode is not adequate for the diagnosis of epilepsy
Autopsy
♦
May have anatomically normal brain (i.e., no seizure focus is identified)
♦
May have mesiotemporal sclerosis within Sommer sector of hippocampus
♦
May find contusion of tongue or pulmonary edema, which are both nonspecific
Microscopic
♦
When present, cell loss may be seen in CA1 and CA4 sectors of hippocampus
Mechanism
♦
May have prolonged tonic-clonic seizures with cardiac and respiratory exhaustion
♦
May have nonvisible seizure with paroxysmal autonomic dysfunction
Bronchial Asthma
Clinical
♦
History of asthmatic bronchitis; death typically occurs during acute asthmatic paroxysm
♦
Death may not correlate with the severity of the autopsy findings
♦
Death more common at night or in early morning
♦
Higher incidence of death in African-Americans than Caucasians (rule out concomitant sickle cell disease trait)
Autopsy
♦
Mucoid plugging of bronchi and edema of mucosa
♦
Voluminous hyperexpanded lungs with indentation by ribs
Microscopic
♦
Prominent mucus in the bronchus, with goblet cells and eosinophils in mucus
♦
Charcot–Leyden crystals, which are derived from major basic protein
♦
Hyaline thickening of basement membrane in bronchial mucosa
♦
Bronchiolar and bronchial smooth muscle and goblet cell hyperplasia
♦
Peribronchial neutrophilic, lymphocytic, and eosinophilic inflammation
Mechanism
♦
Decreased oxygenation and elevated carbon dioxide retention
♦
Increased pulmonary vascular resistance, metabolic acidosis
♦
Eventual exhaustion and death
Bacterial Meningitis
Clinical
♦
Headache and neck stiffness
♦
Common etiologic agent is Streptococcus pneumoniae
Autopsy
♦
Purulent meninges (Fig. 7.7A-D)
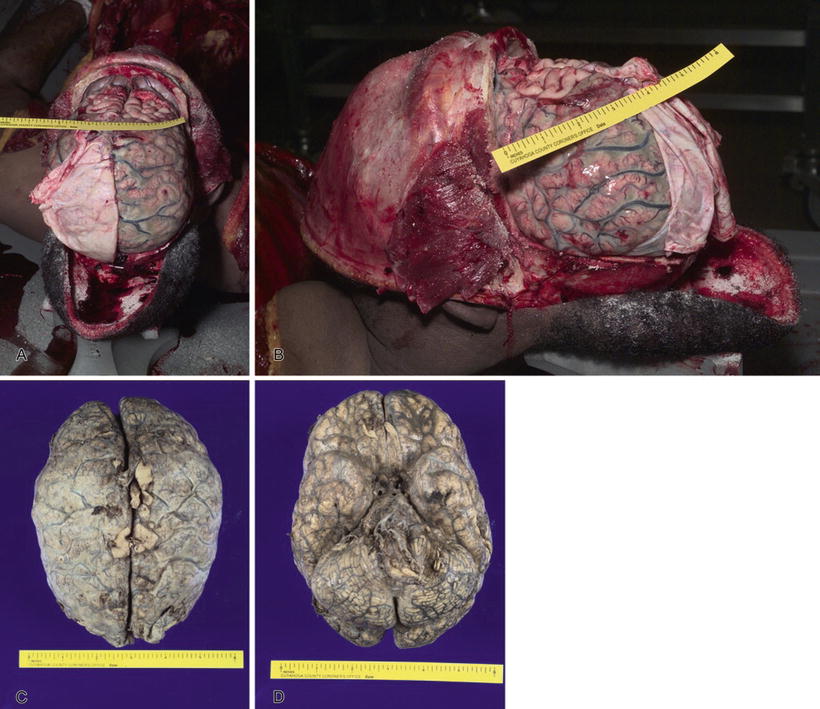

Fig. 7.7
Purulent bacterial meningitis (A). Bacterial meningitis (B). Bacterial meningitis, fixed brain, superior view (C). Bacterial meningitis, fixed brain, inferior view (D).
Microscopic
♦
Extensive neutrophilic infiltrate underneath the arachnoid
Mechanism
♦
Local effects of the inflammatory process and the presence of the bacterial organism
Waterhouse–Friderichsen Syndrome
Clinical
♦
Toxic febrile illness of acute onset with rapid deterioration classically seen associated with Neisseria meningitides meningitis
Autopsy
♦
Bilateral adrenal hemorrhage
♦
Skin changes ranging from petechiae to purpura
♦
Cerebral hemispheres may or may not be visibly suppurative
Microscopic
♦
Adrenal glands with varying degrees of hemorrhage
♦
Affected skin and adrenal glands may show acute neutrophilic infiltrate
Mechanism
♦
Bacterial toxemia and adrenal insufficiency leading to catastrophic rapid onset of shock
Colloid Cyst of Third Ventricle
Clinical
♦
Sudden episodes of headache associated with position of the head
♦
Appearance in adult life
Autopsy
♦
Sudden acute hydrocephalus due to obstruction of foramen of Monroe
♦
1–4 cm gelatinous and hyaline cyst in the anterior region of the third ventricle
♦
Cerebral edema
Microscopic
♦
Epithelial layer of collagenous capsule
♦
Mucus goblet cells present
♦
Stains for mucin with mucicarmine and PAS are positive
Mechanism
♦
Acute hydrocephalus with herniation and compression of brainstem
Diabetic Ketoacidosis
Clinical
♦
History of diabetes mellitus in most cases; however, onset may precede formal diagnosis of disease
Autopsy
♦
May be unremarkable
♦
Large amounts of glucose and acetone, often detected in vitreous humor
♦
Blood may contain acetone
Microscopic
♦
Subnuclear vacuolation in renal proximal convoluted tubule epithelial cells (i.e., Armanni–Ebstein lesion)
♦
Other findings associated with diabetes mellitus are Kimmelstiel–Wilson glomerulopathy and pancreatic islet amyloidosis
Mechanism
♦
Metabolic acidosis, dehydration, and rarely cerebral edema
Anaphylaxis
Clinical
♦
Sudden onset of shortness of breath and edema of face, hives, and vascular collapse
♦
May develop from insect bites, medication, or food
Autopsy
♦
Edema of epiglottis and airway obstruction
Microscopic
♦
Edema and eosinophilic infiltration in airways occasionally
♦
Hypereosinophilia within vasculature of liver and heart
Mechanism
♦
Cardiovascular collapse with sudden onset of shock from systemic vasodilatation that includes pulmonary edema and obstructive angioedema of upper airway
Toxicology
♦
Elevated total tryptase level with beta-tryptase level >1 ng/mL, indicating mast cell degranulation
Manner of Death
♦
Manner of death may be determined to be either natural or accident, depending upon the preference of the forensic pathologist certifying the death
Sudden Infant Death Syndrome
♦
Description: Sudden death of infant <1 year of age, which remains unexplained after performance of a complete postmortem investigation, including review of the case history, examination of the scene of death, and complete autopsy with toxicologic studies and metabolic screening
♦
Based upon the degree of postmortem investigation (e.g., whether or not toxicologic analysis and/or blood cultures are performed), SIDS may be classified into one of three categories, each indicating the extensiveness of investigation prior to the determination of SIDS
♦
In the past, and most likely currently, the diagnosis of SIDS has been used in a wastebasket fashion, with many infants being improperly diagnosed as having died from SIDS, when a subsequent autopsy revealed a definitive cause of death, sometimes even inflicted injury
♦
Some forensic pathologists have recently began to advocate for discontinuing the use of the term “sudden infant death syndrome” and favor certifying all infant deaths without an identifiable cause as undetermined cause and undetermined manner of death
Clinical
♦
SIDS/infant deaths with no identifiable cause of death represent 10–12% of deaths in the first year of life
♦
Majority of deaths occur between 2 and 4 months of age
♦
Incidence is 1–2 per 1,000 infants
♦
Recurrence rate in a family is ~1–2%
Risk Factors Associated with SIDS
♦
Many infant deaths occur while the infant is bed sharing with an adult, sibling, or even possibly pets. In deaths of this manner, an accidental overlay is one consideration, especially when the adult is obese or intoxicated. As the circumstances indicate a possible nonnatural cause of death (i.e., smothering), the criteria for SIDS would not be fulfilled, and the determination of an undetermined cause and manner or other appropriate wording to indicate the possibility of an accidental overlay would be appropriate. Definitive diagnosis of an overlay by autopsy alone, without good corroborating scene investigation, is extremely difficult and most often cannot be made
♦
Maternal risk factors associated with SIDS:
Related to pregnancy: poor prenatal care, low weight gain in pregnancy, multiparity at a young age, and lack of breastfeeding
Unmarried mother
Low education level
Tobacco and drug use
Maternal anemia
♦
Infant risk factors associated with SIDS:
Demographics: male gender, African-American or Native American ancestry
Related to pregnancy: prematurity, small-for-gestational age infant, low birth weight, low Apgar scores (<7), and infant of twin birth
Respiratory distress, tachycardia, tachypnea, cyanosis, fever, or irritability preceding
Hypothermia
Poor feeding
Prone sleeping position: public campaigns to promote putting infants to sleep on their back have led to a decreased incidence of SIDS; however, changes in infant death classification (i.e., more use of overlay and asphyxia as a cause of death) has also resulted in a decreased number of SIDS deaths in some locations
Pathogenesis
♦
Theories proposed include:
Congenital apneic spells or abnormal respiratory control
Brainstem dysfunction
Abnormal sleep and arousal patterns
Upper airway or small airway disease
Cardiovascular abnormalities
Undetected metabolic defects
Abnormal temperature regulation
Infections that are undetected, especially botulism
Developmental vulnerability
♦
The preventative measure suggested is putting an infant to sleep on its side or back instead of on its stomach
♦
SIDS very likely represents a group of disorders that have not yet been delineated as causes of sudden death in infants, and metabolic disorders and unapparent viral syndromes may comprise a significant part of this group
♦
A very small number of infant deaths diagnosed as due to SIDS are undoubtedly concealed homicides, particularly smotherings, therefore potentially one of the reasons behind certifying all such infant deaths (i.e., those with no identifiable cause) as undetermined cause and manner of death
Autopsy
♦
Petechiae of the thymus, pleura, or epicardium
♦
Gliosis of the brainstem and central nuclei
♦
Pulmonary edema or intra-alveolar hemorrhage
♦
Pulmonary hemosiderosis has been described
♦
Histologic evidence of recent viral illness
♦
Extramedullary hematopoiesis
♦
Increased amounts of brown fat in periadrenal adipose tissues
Manner
♦
SIDS is a death presumably due to natural causes
Inborn Errors of Metabolism Resulting in Sudden Death
♦
Disorders of β-oxidation of fatty acids
♦
Glycogen storage disorders
♦
Other disorders
General Features of Disorders of β-Oxidation of Fatty Acids
♦
Fatty acids are an important source of energy for neonates
♦
Enzymes necessary to carry out β-oxidation can be developmentally immature and inadequate in the perinatal period
♦
Infants usually present with sudden death or hypoketotic hypoglycemia
♦
Collapse of metabolic pathways due to concurrent illness or physiologic stress can precipitate symptoms after depletion of hepatic glycogen stores
♦
Myopathy, cardiomyopathy, and liver dysfunction result from the accumulation of fatty acids in the mitochondria and microsomes
♦
Fatty changes of the liver are indistinguishable from Reye syndrome
Specific Disorders of β-Oxidation of Fatty Acids
♦
Medium-chain acyl-CoA dehydrogenase deficiency (MCAD):
MCAD is the first step in fatty acid oxidation:
•
MCAD deficiency may represent the actual cause of death in 1–10% of infant deaths certified as SIDS
•
Autosomal recessive disorder
•
Carrier rate may be as high as 1 in 50 people, resulting in an incidence of 1 in 2,500 to 1 in 7,000 individuals
•
Two known DNA point mutations at positions 985 (A–G) and 583 (S–A)
Diagnosis:
•
Elevated levels of CTs-4-decanoic acid and dodecanoic acid in postmortem fluids
•
Organic acid analysis in urine or vitreous fluid
•
Fatty acid oxidation assay in fibroblasts, liver homogenate, or cells obtained at amniocentesis
♦
Long-chain acyl-CoA dehydrogenase deficiency (LCAD):
Inability to metabolize fatty acids longer than 12–14 carbons
♦
Short-chain acyl-CoA dehydrogenase deficiency (SCAD):
Inability to metabolize fatty acids smaller than six carbons
♦
Multiple acyl-CoA dehydrogenase deficiency (MADD):
Inability to metabolize fatty acids regardless of length
Disorders of Carnitine Metabolism
♦
General features:
Carnitine is a required cofactor of fatty acid oxidation
Low maternal carnitine can result in deficiency in the neonate
Sudden death has been reported in infants with carnitine deficiency in the plasma membrane
Specific enzyme deficiencies:
•
Carnitine palmitoyl transferase type 1 deficiency
•
Carnitine palmitoyl transferase type 2 deficiency
•
Carnitine palmitoyl translocase deficiency
♦
Diagnosis:
Carnitine levels in blood
Bile acylcarnitine levels
Palmitoyl-carnitine oxidation assay using cultured fibroblasts
♦
Therapy for carnitine-related disorders
Carnitine supplementation
Diet modification
Glycogen Storage Disorders
♦
Myophosphorylase deficiency (McArdle disease)
♦
Glycogen storage disease 1a (glucose-6-phosphatase deficiency)
♦
Glycogen storage disease 1b (transport protein T1 deficiency)
♦
Glycogen storage disease 1c (transport protein T2 deficiency)
Other Disorders
♦
Lactic acidemias
♦
Aminoacidopathies
♦
Disorders of carbohydrate metabolism
♦
Hyperglycinemia
♦
Urea cycle defects
Physical Injuries
Mechanical Trauma
♦
When force applied to any part of the body results in harmful disturbance of function or structure
Principles and Effects
Amount of Force
♦
Wound-producing capacity of kinetic energy is determined by weight and velocity:
Formula: KE = MV 2/2g
•
M = weight (mass)
•
V = velocity
•
g = acceleration of gravity
♦
Kinetic energy of a moving object increases proportionally with weight, but exponentially with velocity (e.g., doubling the weight of a bullet doubles the kinetic energy, but doubling the velocity quadruples the kinetic energy)
♦
Further energy may be present if a rotational component exists, again best demonstrated in bullets:
Formula: RE = Mr 2/2g
•
M = weight (mass)
•
r = cross-section radius
•
g = acceleration of gravity
Or RE = IW 2/2g
•
I = rotational inertia
•
W = angular velocity in radians/s or W = 2 × number of rotation/s
•
g = acceleration of gravity
Rate of Energy Transfer
♦
The shorter the duration of impact, the greater energy transfer and the greater the potential for injury
Surface Area
♦
The larger the area through which force is transmitted, the less the intensity and potential for injury
Target Area
♦
Some tissues are more fragile than other tissues, and a fixed amount of force applied to one tissue may cause no injury versus the same force applied to another tissue
Local Effects of Mechanical Injury
♦
Hemorrhage
Once bleeding begins, it will continue until thrombosis, vasoconstriction, or equalization of intravascular and extravascular pressure occurs
The rate and location of hemorrhage is important. A rapid rate of accumulation in certain locations may be lethal (e.g., rapid accumulation of 150 mL of blood in the pericardium or 50 mL of blood in the intracranial cavity may be lethal); however, hemorrhage in the same location at a slower rate may allow the body to adapt and thereby tolerate greater accumulations prior to symptom development
♦
Aseptic inflammation: noninfected inflammatory response to injury
♦
Local infection occurs when the protective layer of tissue is injured and an infectious agent is carried into the wound during the traumatic event or at a later time
Systemic Effects of Mechanical Injury
♦
Primary shock:
Due to an event (e.g., dilatation of rectum, puncture of pleura, pressure to carotid sinuses) that triggers reflex vasodilatation, which causes decreased blood pressure and loss of consciousness
♦
Secondary shock:
Can be due to excessive reduction of blood volume (e.g., hypovolemic shock)
The amount of hemorrhage necessary to produce circulatory collapse is governed by the rate of loss, with more rapid loss more likely to cause adverse results
Rapid loss of one-third of the blood volume leads to hemorrhagic shock
Rapid loss of one-half of the blood volume leads to death
♦
Shock injuries to organs:
Shock kidney: mechanism is ischemic damage due to shunting of blood from the cortex to medulla
Shock lung: morphologic features are hyaline membranes, alveolar and interstitial edema, and interstitial inflammation
♦
Thromboembolism:
A thrombus may form as the result of direct venous injury or from stasis of blood flow due to edema or inactivity
The thrombus may detach, resulting in fatal pulmonary thromboembolism, if sufficient (i.e., >60%) obstruction of the pulmonary arterial circulation occurs
♦
Fat embolism:
Occurs with fracture of long bones and injury to adipose tissue
♦
Fat embolism syndrome:
Clinical findings: the characteristic triad of symptoms is progressive pulmonary insufficiency, petechial rash, and mental deterioration usually occurring 1–3 days following injury, but an individual may also have fever, tachycardia, and renal failure
Ninety to 100% of cases occur associated with major fractures, such as from motor vehicle collisions
Usually lethal when brain involved and with massive fat embolism to the lung, arteriovenous anastomoses in the lung, tearing of lung parenchyma, or interatrial or interventricular defect
♦
Air embolism:
Air enters into dilated veins of gravid uterus during orogenital intercourse or instrumentation, through incision or laceration of veins (especially neck region), disconnection of catheters, or intraoperative procedures of posterior cranial fossa
Air enters the right side of the heart, forming air lock; X-ray of the chest may reveal the right heart filled with air
Air may enter the left side of the heart as the result of an injury to the lung, and air may then embolize to the cerebral or coronary arterial circulation
Blunt Force Injuries
Abrasion
♦
A superficial scrape or scratch injuring the upper layers of skin:
Types of abrasions:
•
Linear abrasion: a scratch
•
Friction abrasion: a brush burn, such as occurs from dragging on a road surface (Fig. 7.8A, B)
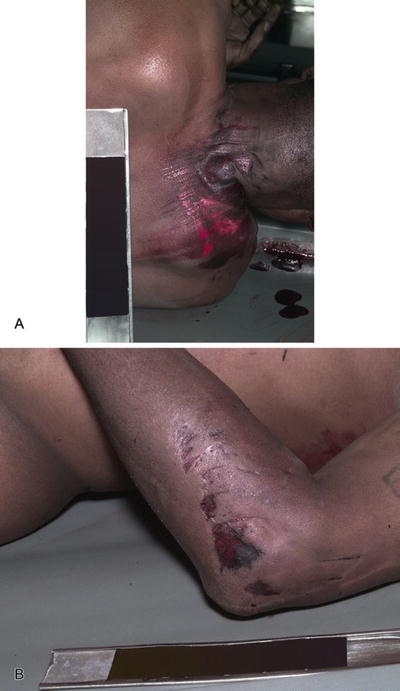
Fig. 7.8
Brush-burn abrasion (A). Brush-burn and linear abrasions (B).
•
Graze: superficial skin injury from a projectile such as a bullet
•
Impact abrasion: can result in a patterned injury that provides information about the object that caused the injury
Abrasions can occur antemortem or postmortem:
•
Postmortem abrasions:
◦
Lack the vital reactions such as hemorrhage, hyperemia, and edema that occur with antemortem injuries
◦
Postmortem loss of epithelium will result in a dried, parchment-like, yellow-tan lesion (Fig. 7.9)
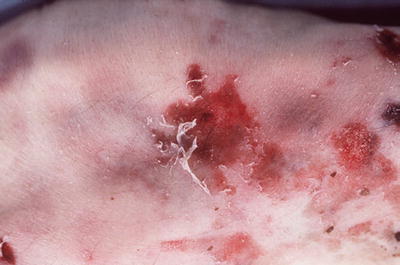
Fig. 7.9
Abrasions with skin margin rolled to distal end.
◦
Postmortem abrasions in areas involved by dependent lividity must be carefully examined
Contusion
♦
An area of hemorrhage into the tissues caused by tearing of blood vessels during blunt trauma:
May be larger or smaller than the impacting object
May be difficult to see in dark-skinned persons
A deep bruise may not appear on the body surface if death follows a short time after the injury
A contusion may appear at a location other than a point of impact
Contusions due to accidents usually involve protuberant or prominent parts of the body, especially areas overlying bone (Fig. 7.10)
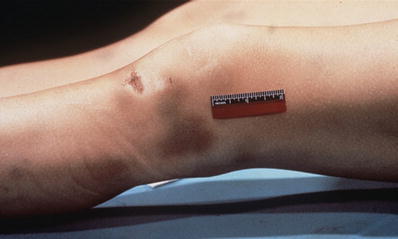
Fig. 7.10
Abrasions and contusions.
Patterned contusions can provide information about the object that caused the injury (Fig. 7.11A, B)
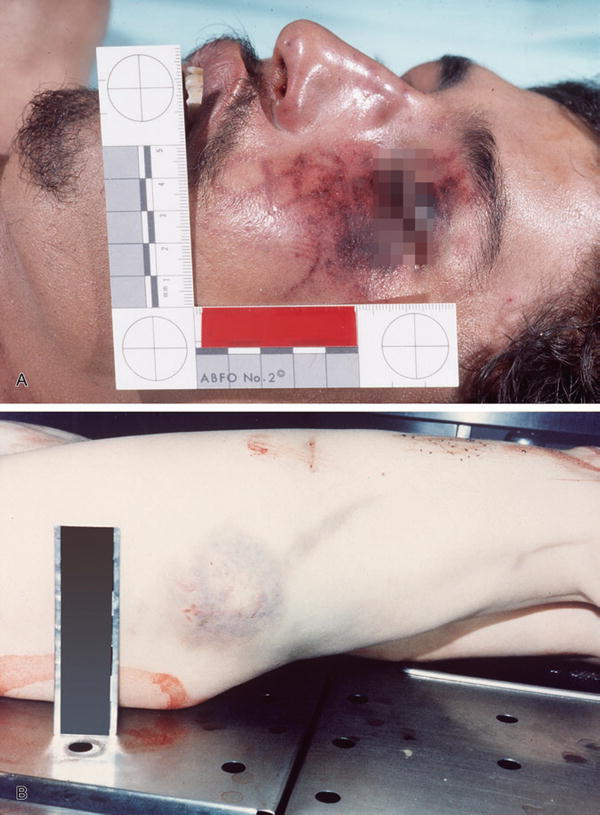
Fig. 7.11
Patterned contusion with ABFO ruler for future pattern comparison (A). Patterned contusion (B).
♦
Dating of contusions:
Contusions resolve from the center outward and from the periphery inward
When multiple contusions are present, they may be due to separate episodes of trauma and, therefore, of differing ages
The coloration of contusions can provide a crude approximation of their age, but contusions of the same age can vary in their degree of resolution in a single individual. Superficial contusions tend to be red, while deeper contusions are more blue or blue purple. The only reliable color indicator for age after onset of the contusion is yellow discoloration due to the presence of hemosiderin, which can begin to appear grossly at 2–5 days after the injury
Iron stains can be used to identify microscopic hemosiderin deposition in an injury that is 24 h old, but hematoidin requires several days to be detected
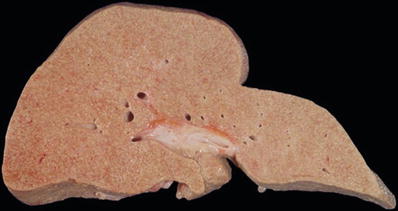
Laceration
♦
A tear produced by a blunt instrument stretching the tissues beyond their elastic tensile strength or crushing and forcible separation of the tissues during compression between two hard surfaces, such as a weapon and the bone underlying the skin
♦
The edges of the tear have abraded margins and ragged edges
♦
There is tissue bridging of the defect by vessels, nerves, and connective tissue (Fig. 7.12A, B)
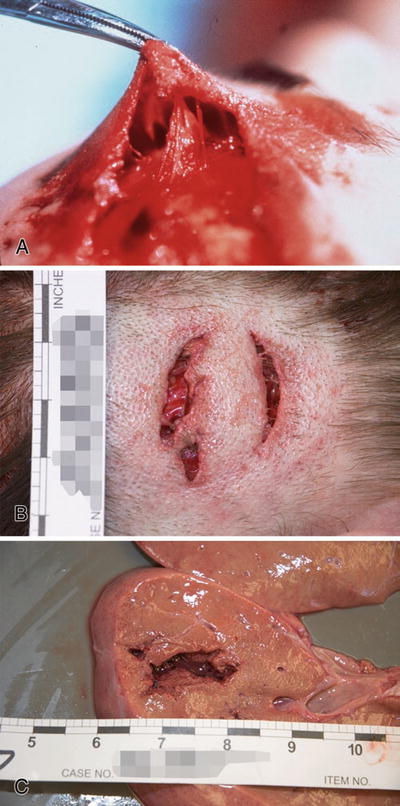

Fig. 7.12
Laceration with prominent tissue bridging (A). Laceration with marginal abrasion (B). Laceration of liver (C).
Fractures
♦
Broken bones due to blunt force injury (Fig. 7.13):
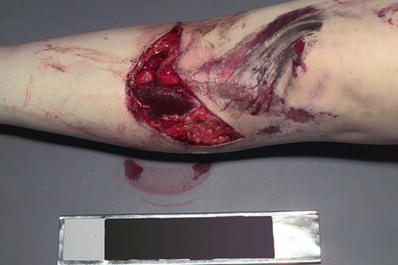

Fig. 7.13
Open tibial fracture with laceration and grease-stained abrasions.
Fractures are produced by either compressive, tensile, or shearing force or a combination of the three, acting on the bone
Special types of fractures:
•
Spiral fractures are due to a twisting motion applied to a long bone of an extremity, usually the legs
•
Classic metaphyseal lesions (CML) result from twisting or wrenching on an extremity, fracturing the bone through the metaphyseal region of the growth plate. Classic metaphyseal lesions in children are highly suggestive of child abuse. Older terms for CMLs, based upon the radiographic appearance of the fracture, are corner, chip, or bucket-handle fractures
•
Avulsion fractures are the result of pulling off the bone attached to a tendon or ligament that supports a joint
Road Traffic Injuries
♦
Motor vehicle accidents can cause massive blunt force injuries:
The most common cause of death is head injury
♦
The tempered side window glass of an automobile produces a special type of injury known as a dicing abrasion:
The tempered glass shatters into small cubes as it breaks and can cause a patterned injury on the side of the face that is nearest the tempered window (Fig. 7.14)
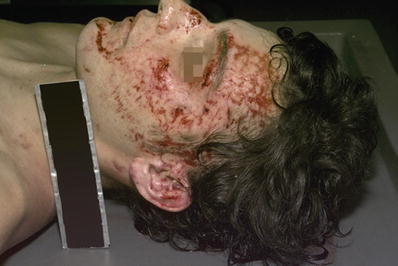
Fig. 7.14
Dicing abrasions of left face – MVA driver.
The safety glass of the front windshield of an automobile is laminated between two layers of plastic and, when it breaks, forms large jagged fragments and not cubes; thus windshields do not cause dicing abrasions
Head Injuries
♦
Blunt force injuries of the head result in several interrelated traumatic lesions
Types of External Scalp Injuries
♦
Get Clinical Tree app for offline access

Lacerations (Fig. 7.15A, B)
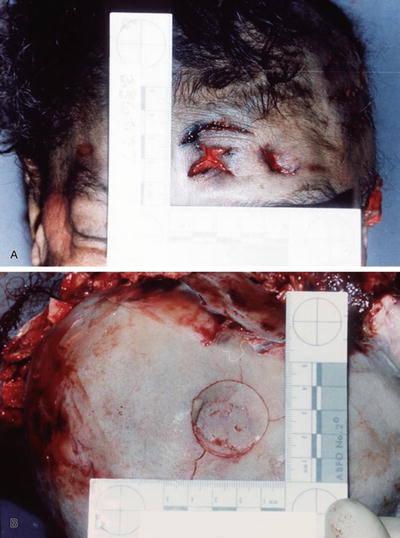

Fig. 7.15




Patterned laceration (A). Patterned depressed skull fracture underlying laceration in Fig. 7.15A (B).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree