=a / (a+b)c / (c+d)
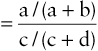
RRR=Disease prevalence in carriers of susceptibility genotypeDisease prevalence in noncarriers of susceptibility genotype

Clinical Validity and Utility
Finding the genetic contributions to health and disease is of obvious importance for research into underlying disease etiology and pathogenesis, as well as for identifying potential targets for intervention and therapy. In medical practice, however, whether to screen individuals for increased susceptibilities to illness depends on the clinical validity and clinical utility of the test. That is, how predictive of disease is a positive test, and how useful is it to have this information?
Clinical Validity
Clinical validity is the extent to which a test result is predictive for disease. Clinical validity is captured by the two concepts of positive predictive value and negative predictive value. The positive predictive value is the frequency with which a group of individuals who test positive have or will develop the disease. For mendelian disorders, the positive predictive value of a genotype is the penetrance. Conversely, the negative predictive value is the frequency with which a group of individuals who test negative are free of disease and remain so. When faced with an individual patient, the practitioner of personalized genetic medicine needs to know more than just whether there is an association and its magnitude (i.e., relative risk or odds ratio). It is important to know clinical validity (i.e., how well the test predicts the presence or absence of disease).
Susceptibility Testing Based on Genotype
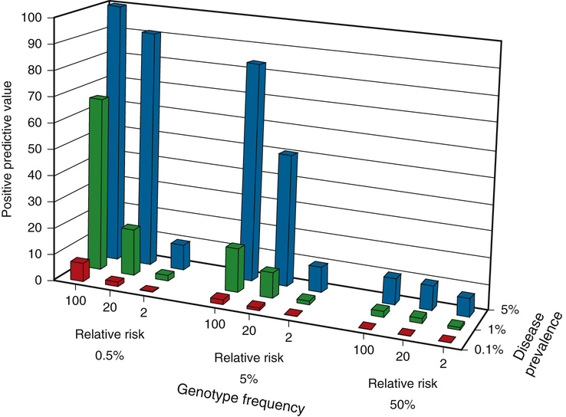
The positive predictive value of a genotype that confers susceptibility to a particular disease depends on the relative risk for disease conferred by one genotype over another and on the prevalence of the disease. Figure 18-4 provides the positive predictive value for genotype frequencies ranging from 0.5% (rare) to 50% (common), which confer a relative risk that varies from low (twofold) to high (100-fold), when the prevalence of the disease ranges from relatively rare (0.1%) to more common (5%). As the figure shows, the value of the test as a predictor of disease increases substantially when one is dealing with a common disorder due to a relatively rare susceptibility genotype that confers a high relative risk, compared with the risk for individuals who do not carry the genotype. The converse is also clear; testing for a common genotype that confers a modest relative risk is of limited value as a predictor of disease.
We will illustrate the use of the 2 × 2 table in assessing the role of susceptibility alleles in a common disorder, colorectal cancer. Shown in the following Box are data from a population-based study of colorectal cancer risk conferred by a polymorphic variant in the APC gene (see Chapter 15) (Case 15) that changes isoleucine to lysine at position 1307 of the protein (Ile1307Lys). This variant has an allele frequency of approximately 3.1% among Ashkenazi Jews, which means that approximately 1 in 17 individuals is a heterozygote (and 1 in 1000 are homozygous) for the allele. The prevalence of colon cancer among Ashkenazi Jews is 1%. The Ile1307Lys variant, common enough to be heterozygous in approximately 1 in 17 Ashkenazi Jews, confers a 2.4-fold increased risk for colon cancer relative to individuals without the allele. However, the small positive predictive value (≈2%) means that an individual who tests positive for this allele has only a 2% chance of developing colorectal cancer. If this had been a cohort study that allowed complete ascertainment of everyone in whom colorectal cancer was going to develop, the penetrance would, in effect, be only 2%.
The Ile1307Lys Allele of the APC Gene and Colon Cancer

• Sensitivity: Fraction of individuals with colon cancer who have the Lys1307 allele = 7/45 = 16%
• Specificity: Fraction without colon cancer who do not have the Lys1307 allele = 4142/4452 = 93%
• Positive predictive value: Fraction of individuals with the Lys1307 allele who develop colon cancer = 7/317 = 2%
• Negative predictive value: Fraction of individuals without the Lys1307 allele who do not develop colon cancer = 99%
Data from Woodage T, King SM, Wacholder S, et al: The APCl1307K allele and cancer risk in a community-based study of Ashkenazi Jews. Nat Genet 20:62-65, 1998.
Clinical Utility
The clinical utility of a test is more difficult to assess than clinical validity, because it has different meanings for different people. In its narrowest sense, the clinical utility of a test is that the result is medically actionable, that is, the result will change what medical care an individual receives and, as a consequence, will improve the outcome of care, both medically and economically. At the other end of the spectrum is clinical utility broadly defined as any piece of information an individual patient might be interested in having, for any reason, including simply for the sake of knowing.
In a patient who tests positive for the APC Ile1307Lys allele, how does a positive predictive value of 2% translate into clinical utility for medical practice? One critical factor is a public health economic one: can the screening be shown to be cost-effective? Is the expense of the testing outweighed by improving health outcomes while reducing health care costs, disability, and loss of earning power? In the example of screening for the APC Ile1307Lys allele in Ashkenazi Jews, more frequent screening or the use of different approaches to screening for colon cancer may be effective. Screening methods (occult stool blood testing versus fecal DNA testing, or sigmoidoscopy versus full colonoscopy) differ in expense, sensitivity, specificity, and potential for hazard, and so deciding which regimen to follow has important implications for the patient’s health and health care costs.
Even with demonstrable clinical validity and actionable clinical utility, demonstrating that testing improves health care is not always straightforward. For example, 1 in 200 to 1 in 250 white individuals are homozygous for a Cys282Tyr mutation in the HFE gene associated with hereditary hemochromatosis, a disorder characterized by iron overload that can silently lead to extensive liver damage and cirrhosis (Case 20). A simple intervention—regular phlebotomy to reduce total body iron stores—can prevent hepatic cirrhosis. The susceptibility genotype is common, and 60% to 80% of Cys282Tyr homozygotes show biochemical evidence of increased body iron stores, which suggests that screening would be a reasonable and cost-effective measure to identify asymptomatic individuals who should undergo further testing and, if indicated, the institution of regular phlebotomy. However, most Cys282Tyr homozygotes (>90% to 95%) remain clinically asymptomatic, leading to the argument that the positive predictive value of HFE gene testing for liver disease in hereditary hemochromatosis is too low to justify population screening. Nonetheless, some of these largely asymptomatic patients do have signs of clinically occult fibrosis and cirrhosis on liver biopsy, indicating that the Cys282Tyr homozygote may actually be at a higher risk for liver disease than previously thought. Thus some would argue for population screening to identify individuals in whom regular prophylactic phlebotomy should be instituted. The clinical utility of such population screening remains controversial and will require additional research to determine the natural history of the disease and whether the silent fibrosis and cirrhosis seen on liver biopsy represent the early stages of what will be a progressive illness.
APOE testing in Alzheimer disease (AD) (see Chapter 12) (Case 4) is another example of the role of a careful assessment of clinical validity and clinical utility in applying genetic testing to personalized medicine. As discussed in Chapter 8, heterozygotes for the ε4 allele of the APOE gene are at a two- to threefold increased risk for development of AD compared with individuals without an APOE ε4 allele. APOE ε4/ε4 homozygotes are at a eightfold increased risk. An analysis of both the clinical validity and clinical utility of APOE testing, including calculation of the positive predictive value for asymptomatic and symptomatic individuals, is shown later (Table 18-5).
TABLE 18-5
Clinical Validity and Utility of APOE Population Screening and Diagnostic Testing for Alzheimer Disease

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



