22 FOLLICLE DEVELOPMENT AND THE MENSTRUAL CYCLE
DEVELOPMENT OF THE FEMALE REPRODUCTIVE TRACT
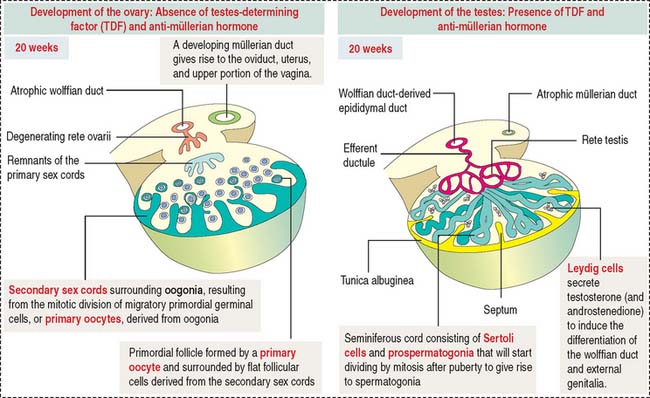
Development of the ovary
As discussed in Chapter 21, Sperm Transport and Maturation, the cortical region of the primitive gonad develops into an ovary. The cortical region of the indifferent gonad initially contains the primary sex cords (fifth week of development).
One week later, cells of the primary cell cords degenerate and are replaced by secondary sex cords that surround individual oogonia (Figure 22-1).
Development of the female genital ducts
The urogenital sinus also gives rise to the urinary bladder, urethra, vestibular glands, and hymen.
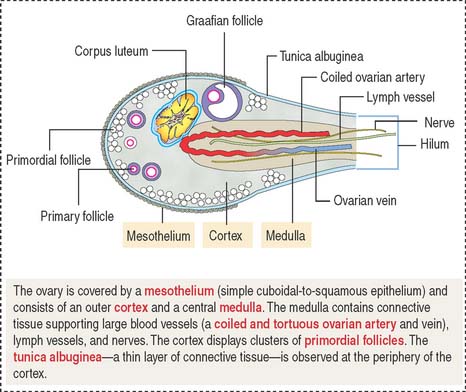
THE OVARIES
Each ovary is lined by a simple squamous-to-low cuboidal epithelium and a subjacent connective tissue layer, the tunica albuginea. In a cross section, a cortex and a medulla without distinct demarcation can be visualized. The broad cortex contains connective tissue and primordial follicles housing primary oocytes (at the end of meiotic prophase I). The medulla consists of connective tissue, interstitial cells, nerves, lymphatics, and blood vessels reaching the ovary through the hilum (Figure 22-2).
Ovarian cycle
The three phases of the ovarian cycle are the follicular phase, ovulatory phase, and luteal phase.
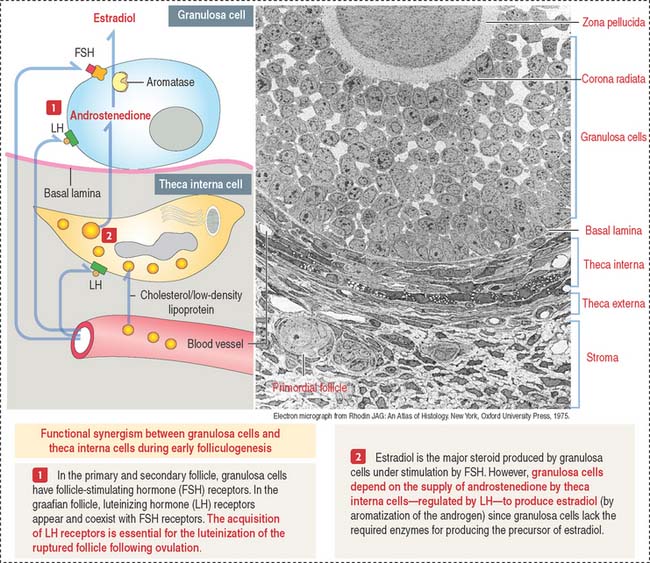
The follicular phase consists of the development of several primordial follicles into one antral, preovulatory or graafian follicle (Figures 22-3 and 22-4) normally selected to ovulate. The time interval from recruitment of primordial follicle cohorts to the development of a preovulatory graafian follicle averages about 3 to 6 months. During this time, follicles acquire follicle-stimulating hormone (FSH), estrogen and androgen receptors and granulosa cells and primary oocytes become functionally coupled by gap junctions.
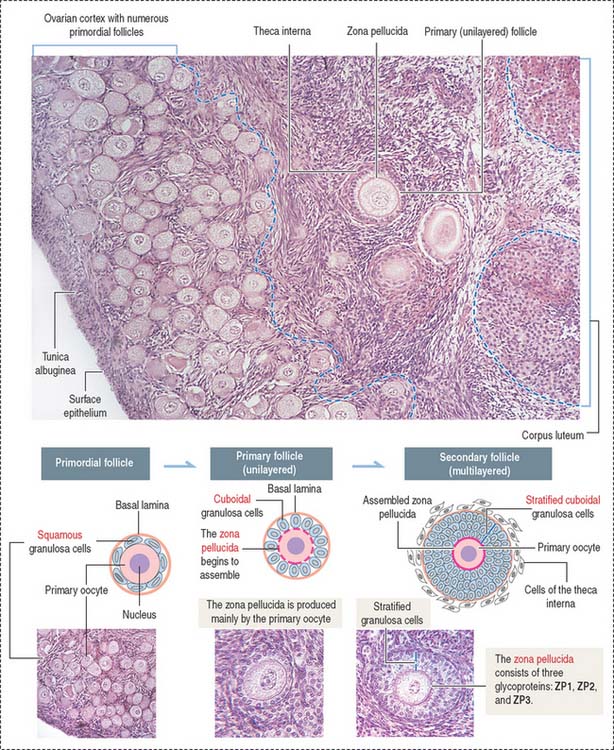
The predominant and smallest follicle (25 μm in diameter) is the primordial follicle, which is surrounded by squamous, flattened granulosa cells (see Figure 22-3).
Primordial follicles leaving the resting phase are called primary follicles. This transition represents a commitment of primary follicles to subsequent stages of follicular development. Primary follicles are lined by a single layer of cuboidal granulosa cells that become proliferative and separated from the stroma of the ovary by a basal lamina.
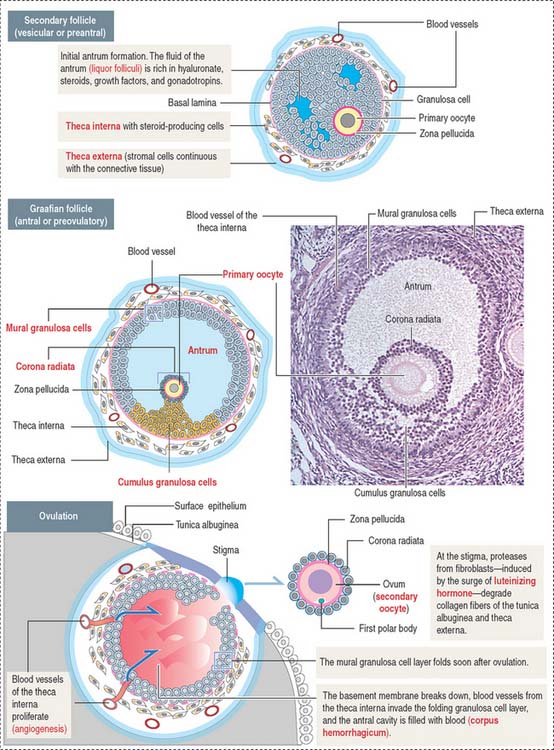
Granulosa cells continue to proliferate into several layers, forming multilayered secondary follicles. Three events characterize the development of secondary follicles: (1) the initiation of the assembly of the zona pellucida; (2) the accumulation of fluid (liquor folliculi) between the granulosa cells; and (3) the distinction of a cellular shell or theca (theca folliculi; Greek theke, box) that separates the ovarian stroma from the granulosa cell multilayer. The theca cells, recruited from surrounding stromal cells, organize into two distinct layers around each follicle: (1) the theca interna and (2) the theca externa.
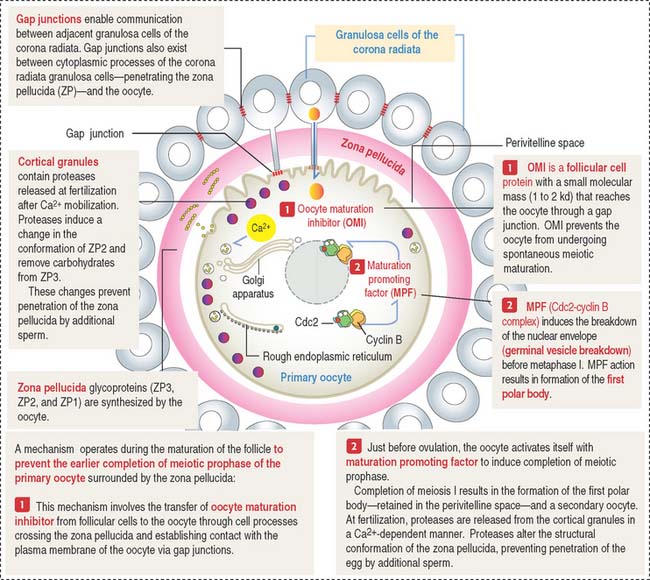
Zona pellucida is a glycoprotein coat that progressively separates granulosa cells from the primary oocyte. The zona pellucida is penetrated by thin cytoplasmic processes of the granulosa cells that contact microvilli of the oocyte. Gap junctions, present at granulosa cell-oocyte contact sites, enable molecular communication that prevents the untimely completion of meiotic prophase of the primary oocyte (Figure 22-5). Gap junctions are also seen between granulosa cells.
During the subsequent developmental phase, the graafian follicle—also known as preovulatory or antral follicle—the formation of the antrum soon segregates the granulosa cells with respect to the primary oocyte into two specific regions: the cumulus granulosa cell region (cumulus oophorous), in close proximity to the primary oocyte, and the mural granulosa cell region lining the wall of the follicle (see Figure 22-4). The layer of granulosa cells firmly anchored to the zona pellucida is referred to as the corona radiata (Figures 22-5 and 22-6). A preovulatory graafian follicle reaches about 20 mm in diameter (as compared to the 25 μm in diameter of a primordial follicle).
The theca externa is a connective tissue capsule-like layer, continuous with the ovarian stroma. In contrast, the theca interna is a well-vascularized cell layer adjacent to the basal lamina of the developing follicle. It consists of elongated cells with small lipid droplets in the cytoplasm acquiring the characteristics of steroid-secreting cells. Cells of the theca interna secrete androstenedione, an androgen precursor that is transferred across the basal lamina to the granulosa cells for the production of testosterone (see Figure 22-6). Testosterone is then converted to estradiol by aromatase. Granulosa cells lack enzymes required for the direct production of estrogens. As a result, granulosa cells cannot produce steroid precursors during folliculogenesis.
Primary oocyte-granulosa cell communication during folliculogenesis
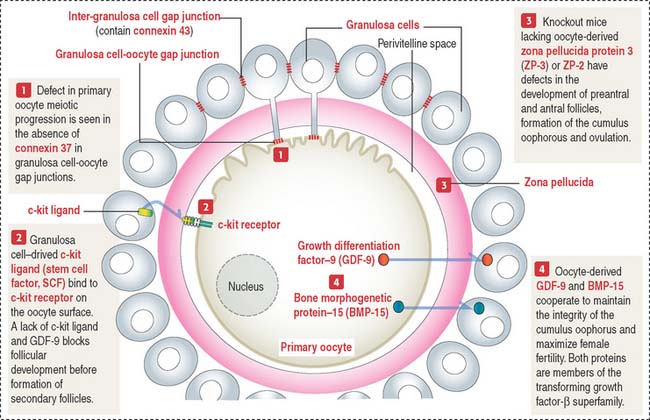
Bidirectional communication between the oocyte and granulosa cells occurs during folliculogenesis. Figure 22-7 provides a summary of the molecular intercellular communication events. Connexin 37 is present in gap junctions linking granulosa cells of the corona radiata and the primary oocyte. Connexin 43 is found in gap junctions connecting granulosa cells. A lack of connexin 37 results in a defect in the capacity of the primary oocyte to resume meiosis and epigenetic modifications essential for fetal development. A lack of connexin 43 disrupts folliculogenesis during the preantral phase.
Granulosa cell-derived factors include c-kit ligand (or stem cell factor ligand) which binds to the oocyte c-kit receptor and stimulates oocyte growth and survival. As you remember, c-kit receptor and its ligand play significant roles in the migration of primordial germinal cells to the gonadal ridges during gonadogenesis (see Chapter 21, Sperm Transport and Maturation).
Box 22-C Ovarian hormones
As you realize, many proteins of the TGF-β superfamily coordinate folliculogenesis and help to produce a competent primary oocyte
Follicular atresia or degeneration
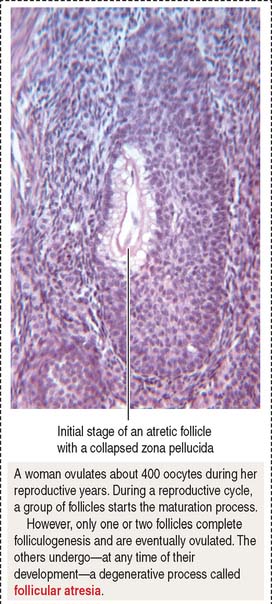
Several primary follicles initiate the maturation process (see Box 22-A), but only one follicle completes its development; the remainder degenerate by an apoptotic process called atresia. Follicles can become atretic at any stage of development.
Box 22-A Polycystic ovary syndrome
Atretic follicles (Figure 22-8) are identified by a thick folded basement membrane material, the glassy membrane, a relatively intact zona pellucida, remnants of degenerated oocytes and granulosa cells, and invading macrophages.