http://evolve.elsevier.com/Edmunds/NP/
Quinolones were discovered in 1962 as a byproduct of chloroquine synthesis. The earliest drugs were called quinolones. Later, a fluorine radical was added to the structure, and these newer drugs were called fluoroquinolones, to distinguish them from the earlier drugs. Currently, all quinolones on the market are fluoroquinolones. Many texts now use the terms quinolone and fluoroquinolone interchangeably.
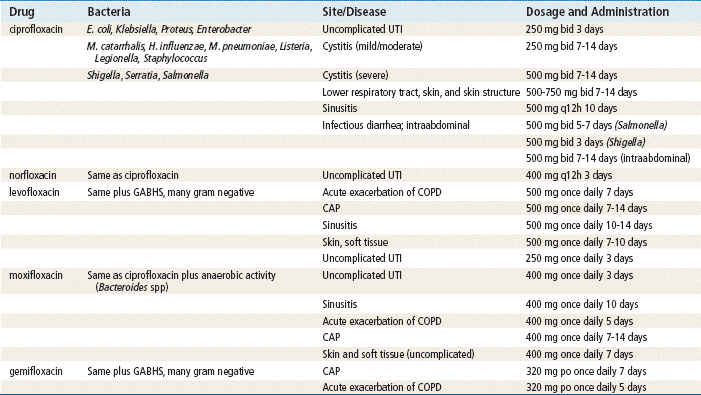
The fluoroquinolones are a group of potent antibiotics with a wide spectrum of action against gram-positive and gram-negative organisms. The primary care provider should be familiar with ciprofloxacin, moxifloxacin, and levofloxacin. Fluoroquinolones have a good safety profile and excellent absorption after oral administration. Headache and dizziness occur in up to 11% of patients and are the most commonly reported adverse events. The newer fluoroquinolones offer expanded coverage of gram-positive pathogens, and one agent, moxifloxacin, is active against anaerobes. However, three fluoroquinolones introduced after 2001 have been taken off the market because of adverse events.
Quinolones are active against aerobic gram-negative bacilli, especially Enterobacteriaceae, Haemophilus, and gram-negative cocci such as Neisseria and Moraxella catarrhalis. They are active against Staphylococcus and Mycoplasma. Ciprofloxacin and levofloxacin are the only quinolones active against Pseudomonas aeruginosa.
Therapeutic Overview
Allergic reactions occur in 0.5% to 3% of patients. Rashes are the most common allergic reaction. The newest fluoroquinolone, gemifloxacin, causes rashes in 14% of young women. More serious reactions such as anaphylaxis, drug fever, vasculitis, interstitial nephritis, and serum sickness have been uncommon. If patients demonstrate any of these reactions, it is recommended that they not take any of the fluoroquinolones.
Resistance
Resistance occurs by mutation. No quinolone-modifying abilities have been seen in bacteria. Spontaneous mutations that result in resistance alter the target enzymes or alter drug transport across the cell wall. Plasmid-mediated quinolone resistance has been seen. These mutations produce efflux pumps to remove the antibiotic from the cell. Pseudomonas and staphylococci are the two most troublesome. Recently, resistance to Neisseria gonorrhoeae, Campylobacter jejuni, and Escherichia coli has been noted. Underdosing caused by failure to finish the prescribed course of medication should be avoided to prevent resistance.
Mechanism of Action
Quinolones have a dual ring structure. Substitutions on the dual rings determine the type of quinolone. Ofloxacin is a racemic mixture (i.e., right- and left-handed molecules) that contains the active ingredient, levofloxacin. The mechanism of action of the fluoroquinolones is inhibition of deoxyribonucleic acid (DNA) gyrase, an enzyme that is essential in the transcription, replication, and repair of bacterial DNA. The blocking of DNA gyrase that occurs at concentrations of 0.1 to 10 g/ml promotes superhelical formation of bacterial DNA, resulting in destruction of the DNA.
Treatment Principles
Fluoroquinolones should not be considered first-line antibiotics for mild infection; however, they are often the agent of first choice for UTIs and pyelonephritis because of the frequency of resistant bacteria with these conditions. In general, they should be reserved for moderate infections and bacterial infections that are resistant to other antibiotics. Because fluoroquinolones have good tissue penetration, they provide effective treatment for skin, bone, and genital infections. CNS penetration, however, is poor and is not considered clinically effective. Always give fluoroquinolones orally when possible because oral absorption is excellent.
Fluoroquinolones are considered first-choice drugs for empirical treatment of complicated sinusitis, severe diarrhea, UTIs, pyelonephritis, and prostatitis. The CDC advises that fluoroquinolones should not be used any longer for gonorrhea. They are acceptable as an alternative treatment for dog bite, acute sinusitis, severe sinusitis, and chlamydia. Fluoroquinolones with enhanced activity via S. pneumoniae provide alternative therapy for acute exacerbation of COPD and pneumonia in adults.
Fluoroquinolones are active against many gram-positive organisms; gram-negative organisms, including M. catarrhalis, Haemophilus influenzae, E. coli, chlamydia, and Mycoplasma pneumoniae; and anaerobes. They are not active against Clostridium difficile.
Warnings have been issued for the fluoroquinolone antibiotics for the increased risk of tendon ruptures. Ruptures have occurred unilaterally and bilaterally, and have involved the Achilles tendon; however, ruptures in the shoulder joint, hand, biceps, thumb, and other tendons sites have been reported. The risk of tendon rupture is further increased in those over age 60, those receiving concomitant steroid therapy, and in kidney, heart, and lung transplant recipients. Reasons for tendon ruptures also include physical activity or exercise, kidney failure, and tendon problems in the past. These ruptures may occur during therapy or up to several months after discontinuation of drugs. There also may be rare risk for serious and potentially fatal adverse events that may occur after repeated doses. Some tendonitis and tendon rupture requires surgical repair or results in prolonged disability. A significant number of cases have been reported, including cases in the United States. Discontinue ciprofloxacin at the first sign of tendon inflammation or tendon pain, as these are symptoms that may precede rupture of the tendon. The products identified with increased risk are second-generation quinolone ciprofloxacin (Cipro, tablets or oral suspension), intravenous injection (Cipro IV), and extended-release tablets (Cipro XR), as well as ofloxacin (Floxin tablets) and the third-generation quinolone levofloxacin (Levaquin tablets, oral solution, injection, and injection in 5% dextrose).
There is the risk for serious and occasionally fatal hypersensitivity reactions that may occur after the first dose of a quinolone. These have included cardiovascular collapse, hypotension or shock, seizure, loss of consciousness, tingling, angioedema, airway obstruction, dyspnea, urticaria, and other serious skin reactions. There is a rare risk for serious and sometimes fatal events after multiple doses. Clinical manifestations may include one or more of the following: fever, rash, or severe dermatologic reactions (e.g., toxic epidermal necrolysis and Stevens-Johnson syndrome); vasculitis, arthralgia, myalgias, and serum sickness; allergic pneumonitis; interstitial nephritis and acute renal insufficiency or failure; hepatitis, jaundice, and acute hepatic necrosis or failure; and anemia (including hemolytic and aplastic), thrombocytopenia (including thrombocytopenic purpura), leukopenia, agranulocytosis, pancytopenia, and other hematologic abnormalities. Treatment should be discontinued at the first sign of a skin rash, jaundice, or any other sign of hypersensitivity, and supportive measures should be instituted.
Treatment with a fluoroquinolone can alter the colon’s normal flora, leading to overgrowth of C. difficile and subsequent release of toxins A and B that contribute to the development of c. difficile acute diarrhea (CDAD). Nearly all antibiotics have been implicated in CDAD, which may range in severity from mild diarrhea to fatal colitis. Because hypertoxin-producing strains of C. difficile can be refractory to antimicrobial therapy, they are associated with increased morbidity and mortality and may require colectomy. The FDA advises that CDAD be considered in all patients who present with diarrhea after antibiotic use. Careful examination of medical history is required because of the potential for late-onset disease; cases of CDAD have been reported more than 2 months after completion of an antimicrobial course of therapy. The FDA notes that current antibiotic therapy for the primary infection may need to be discontinued in patients with known or suspected CDAD. Appropriate fluid and electrolyte management, protein supplementation, antibiotic therapy for C. difficile, and surgical evaluation also may be required.
In a recently published case-controlled study, it was found that levofloxacin and moxifloxacin, two commonly used fluoroquinolones, are tied to higher odds of hospitalization among older adults for idiosyncratic liver injury. Researchers said the chance of hospital admission is greater with the drugs compared with clarithromycin. It is not clear yet whether these drugs will be given regulatory warnings by the FDA.
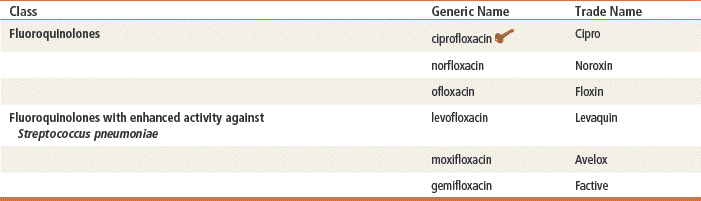
 Key drug.
Key drug.