INTRODUCTION
FLUIDS & ELECTROLYTES
Surgical patients are at high risk for derangements of body water distribution, electrolyte homeostasis, and acid-base physiology. These disturbances may be secondary to trauma, preexisting medical conditions which alter normal physiology, or the nature of the surgery.
Total body mass is 45%-60% water. The percentage in any individual is influenced by age and lean body mass, therefore the percentage is higher in men compared to women, in children compared to adults, and in people of normal body habitus compared to the obese (Table 9–1). Two-thirds of total body water (TBW), 30%-40% of body mass, is intracellular; one-third, 15%-20% of total body mass, is extracellular. The extracellular fluid is divided into two compartments, with 80% (12%-16% of total body mass) in the interstitial compartment, and 20% (3%-4%) in the intravascular compartment. One-fifth of intravascular fluid is proximal to the arterioles, the remaining four-fifths is distal to the arterioles.
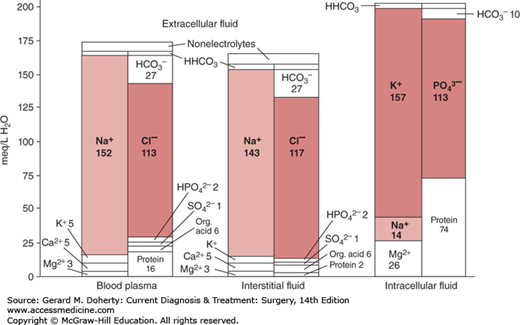
The intracellular, interstitial, and intravascular compartments each hold fluid characterized by markedly different electrolyte profiles (Figure 9–1). The main intracellular cation is the potassium ion (K+), while the main extracellular cation is the sodium ion (Na+). Not only the electrolyte profile, but also the protein composition of the fluids differs: intracellular cations are electrically balanced mainly by the polyatomic ion phosphate (PO43−) and negatively charged proteins, while extracellular cations are balanced mainly by the chloride ion (Cl−). The intravascular fluid has a relatively higher concentration of protein and lower concentration of organic acids than the interstitial fluid. This higher concentration of protein, chiefly albumin, is the main cause of the high colloid osmotic pressure of serum, which in turn is the chief regulator of the fluid distribution between the two extracellular compartments. The relationship between colloid osmotic pressure and hydrostatic pressure governs the movement of water across the capillary membrane, and is modeled by the Starling equation.
The body’s volume status and electrolyte composition are determined largely by the kidneys. The kidneys maintain a constant volume and osmolality by modulating how much free water and Na+ is reabsorbed from the renal filtrate. Antidiuretic hormone (ADH), also known as arginine vasopressin, is the chief regulator of osmolality. The peptide hormone is released from the posterior pituitary in response to increased serum osmolality. ADH induces translocation of aquaporin channels to the collecting duct epithelium, increasing permeability to water and causing reabsorption of free water from the renal filtrate. Thus water is retained, and the urine concentrated. In the absence of ADH, the collecting duct is impermeable to water, leading to water loss and production of dilute urine. At high physiologic levels ADH has a direct vasoconstrictive effect on arterioles.
The main determinant of Na+ reabsorption is the Na+ load in the renal filtrate. Most filtered Na+ (60%-70%) is reabsorbed in the proximal tubule. A further 20%-30% of filtered Na+ is reabsorbed in the thick ascending limb of the loop of Henle; reabsorption here is determined by the Na+ load delivered to the loop and a variety of hormones. The remaining distal tubule reabsorbs 5%-10% of filtered Na+; again, the exact percentage is determined by the Na+ load and a variety of hormonal factors, particularly aldosterone. The collecting duct reabsorbs a small percentage of filtered Na+ under the influence of aldosterone and natriuretic hormone. Under normal circumstances the kidneys will adjust excreted water and Na+ to match a wide spectrum of dietary intake.
Although the movement of ions and proteins between the different fluid compartments is normally restricted, water itself is freely diffusible between them. Consequently the osmolality of the different fluid compartments is identical, normally approximately 290 mOsm/kg. Control of osmolality occurs through regulation of water intake through diet and excretion through urine and insensible loses.
VOLUME DISORDERS
Hypo- and hypervolemia commonly occur in surgical patients, both following elective surgery and in the setting of trauma and acute care surgery. Volume disturbances run the gamut from being clinically insignificant to being immediately life threatening. The underlying cause of any volume disorder must be sought and addressed while the volume disorder itself is managed.
Hypovolemia
Etiology: Hypovolemia is common in surgical patients. It is typically caused by loss of isotonic fluids in the setting of hemorrhage, gastrointestinal losses (eg, gastric suctioning, emesis, and diarrhea), sequestration of fluids in the gut lumen (eg, bowel obstruction, ileus, and enteric fistulas), burns, and excessive diuretic therapy. In resource poor settings sweat is often an additional important fluid loss, for example, in a non–air-conditioned operating room. In all of these cases, loss of isotonic fluid results in loss of Na+ and water without significantly affecting the osmolality of the extracellular fluid compartment, thus there is very little shift of water into or out of the intracellular compartment. Hypovolemia stimulates aldosterone secretion from the zona glomerulosa of the adrenal cortex, leading to increased reabsorption of Na+ and water from the renal filtrate and excretion of low volumes (oliguria) of hypertonic urine with a low Na+ concentration.
Fractional excretion of Na+ (FENa) is a useful tool for differentiating causes of oliguria:
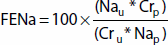
where U: urine, P: plasma, Na: sodium, Cr: creatinine.
FENa ≤1% usually indicates prerenal azotemia. However, FENa ≤1% may be found in patients with oliguria secondary to hepatorenal syndrome, as the liver does not effectively metabolize aldosterone. In patients already receiving diuretic therapy, fractional excretion of urea nitrogen (FEUN) is more helpful than FENa. FEUN is calculated analogously; FEUN ≤35% indicates prerenal azotemia.
Presentation: Hypovolemia is suggested by a patient’s history, physical examination, and laboratory data. Diagnosis by physical examination alone in the immediate postoperative setting is difficult, especially when volume loss is mild to moderate, and especially in very old and very young patients. One review found that longitudinal furrows on a patient’s tongue and dry oral and nasal mucous membranes are 85% sensitive for hypovolemia; increased capillary refill time, unclear speech, upper or lower extremity weakness, a dry axilla, and postural hypotension were all relatively specific indicators of hypovolemia. The same review found that a postural (supine to standing) increase in heart rate of at least 30 beats per minute or severe postural dizziness which prevented the patient from standing had 6%-48% sensitivity for mild to moderate hypovolemia but 91%-100% sensitivity for severe hypovolemia. Laboratory evidence of hypovolemia includes elevated blood urea nitrogen (BUN): creatinine (Cr) ratio, as hypovolemia decreases renal perfusion, causing prerenal azotemia characterized by a disproportionate rise in BUN compared to Cr. However, an elevated BUN: Cr ratio may also be associated with renal failure and with gastrointestinal bleeding, independent of volume status. No single highly sensitive test to diagnose hypovolemia exists, thus the diagnosis must be made by examining all available data with a high index of suspicion.
Treatment: Hypovolemia is corrected with intravenous administration of an isotonic fluid. Coexistent electrolyte abnormalities should be addressed simultaneously. Care must be taken in patients in renal or heart failure not to exacerbate these conditions. If hypovolemia is allowed to worsen unimpeded it will eventually lead to circulatory collapse and shock, which is covered elsewhere.
Hypervolemia
Etiology: Hypervolemia is also common in surgical patients. It often occurs after treatment of shock with colloid and crystalloid fluids, with or without attendant renal failure. It also occurs in the postoperative period as ADH is secreted in response to nonphysiologic stimuli, disrupting its role in regulation of osmolality. High physiologic levels of ADH have a vasoconstrictive effect, leading to a decreased filtered Na+ load and thus greater Na+ and water retention. This secretion of ADH typically ceases 2-3 days after the surgical insult, after which ADH levels return to an appropriate level and patients experience a so-called “autodiuresis.” Heart failure, liver disease, renal disease, and malnutrition all exacerbate hypervolemia in the surgical patient. Preexisting heart failure decreases the range across which a patient will adequately compensate for increased intravascular volume. Liver disease decreases metabolism of circulating ADH and aldosterone, increasing the homeostatic set point for intravascular volume and thus decreasing the physiologic reserve remaining to respond to surgical insults. Renal disease disrupts all aspects of volume regulation. Syndrome of inappropriate ADH hypersecretion (SIADH) causes hypervolemia secondary to a sustained ADH release independent of the normal physiologic triggers. SIADH typically occurs in the setting of traumatic brain injury, brain tumors and abscesses, pneumonia and lung abscesses, as a paraneoplastic syndrome associated with small cell lung cancer and other neoplasias, and with a variety of drugs including morphine and the chemotherapeutic agents cyclophosphamide and vincristine.
Presentation: Like hypovolemia, hypervolemia is often suggested by the patient’s history and physical examination. Signs include hypertension, decreased arterial oxygen saturation and basilar crackles, jugular venous distention, dependent soft tissue edema, gallop rhythms on cardiac auscultation, and rapid weight gain. Examination of recorded fluid administration may assist in the diagnosis. Eventually a hypervolemic patient may show signs of pulmonary edema, easily diagnosed on chest x-ray, or even congestive heart failure (CHF).
Treatment: If the hypervolemic patient develops CHF or pulmonary edema, treatment with diuretics, or in extreme cases hemodialysis, may be required. Mechanical ventilation may be lifesaving if pulmonary edema and/or CHF have progressed to the point of respiratory failure.
Electrolyte derangements may occur independently of each other, but are often closely linked. Like volume disorders, disturbances of electrolyte homeostasis run the gamut from mild and inconsequential to severe and immediately life threatening.
The underlying cause of any electrolyte disorder must be sought while the electrolyte disorder itself is managed. Special care must always be taken when repleting electrolytes in patients with renal insufficiency. These patients require slower repletion and more frequent monitoring of electrolyte levels.
Sodium: Hypo- and hypernatremia reflect excessive gain or loss of TBW, respectively. Significant derangements of Na+ homeostasis result in significant changes to plasma tonicity and eventually whole body osmolality, with potentially devastating consequences for the central nervous system (CNS).
Hyponatremia
Etiology: Hyponatremia is a sign of relative water gain; total body Na+ may be decreased, normal or even increased. Hyponatremia is common in surgical patients postoperatively, as ADH is secreted in response to pain, nausea and vomiting, opiate administration, and positive-pressure ventilation. Typically, this hyponatremia is mild and inconsequential; however, it may be exacerbated by rapid parenteral administration of hypotonic fluids. Hyponatremia may result from severe hyperglycemia, or any other condition in which an osmotically active solute draws water from the intracellular space to the extracellular space. In the setting of hyperglycemia, a corrected Na+ is calculated as:
Na+corrected = Na+measured + 0.016 (plasma glucose in mg/dL − 100)
The correction factor 0.016 accounts for the unit conversion from mg/dL to mmol/L. Some investigators believe a correction factor of 0.024 is more appropriate.
Other causes of hyponatremia include cerebral salt-wasting syndrome, liver disease, congestive heart failure, and SIADH.
Presentation: The signs of hyponatremia are caused by CNS dysfunction, as brain cells swell in a fixed-volume space (the Monro-Kellie hypothesis). Mental status changes, obtundation, coma, and seizures typically are not seen until serum Na+ is less than 120 mmol/L. Such hyponatremia is a medical emergency requiring immediate intervention.
Treatment: CNS signs and symptoms attributable to hyponatremia require administration of normal saline and free water restriction. In general, hypertonic saline should not be used to correct hyponatremia. Unless serum Na+ falls very rapidly, it should be corrected slowly, as too-rapid correction has the devastating complication of osmotic demyelination. No consensus exists on the appropriate rate of correction. One review recommended correction by no more than 8 mmol/L/d, starting with correction of 1-2 mmol/L/h in patients with severe manifestations. Sodium levels should not be corrected beyond what is needed to alleviate CNS disturbances. Correction of Na+ does not replace pharmacologic intervention in a seizing patient.
One formula used to calculate the expected change in Na+ from intravenous administration of 1 L of any fluid is:

Total body water is estimated as a fraction of body mass (Table 9–1). Hyponatremia causing CNS disturbances should prompt admission to an intensive care unit given the close monitoring needed in such patients, the need for rapid intervention, and the potentially devastating consequences of delays in management.
Hypernatremia
Etiology: Loss of free water alone occurs when patients do not have access to water (eg, preverbal, bed-bound or otherwise incapacitated patients), in diabetes insipidus, in the setting of high fevers, and in patients in whom enteral feeds do not contain adequate water. Hypernatremia typically occurs simultaneously with volume derangements, and total body Na+ may be increased, normal or even decreased. Induced hypernatremia is a useful treatment modality in patients with traumatic brain injury to reduce cerebral edema, decrease intracranial pressure, and increase cerebral perfusion pressure.
Presentation: As with hyponatremia, the signs and symptoms of hypernatremia are caused by CNS dysfunction: lethargy, fatigue, hyperactive deep tendon reflexes, seizure, and coma may occur. Signs and symptoms are rare with Na+ less than 158 mmol/L.
Treatment: Development of CNS symptoms requires parenteral administration of free water, typically as 5% dextrose in water. If the patient’s hypernatremia developed over a period of hours it may safely be corrected at a rate of 1 mmol/L/h. In patients whose hypernatremia developed more slowly, a rate of 0.5 mmol/L/h is safe. As derangements in Na+ are caused by derangements in TBW, the concept of a “water deficit” is a useful one in treating hypernatremia. The free water deficit is calculated as:
water deficit = (TBW × (1 − [140/Na+ in mmol/L])
This formula is accurate when calculating the water deficit in patients with pure water loss, but is inaccurate in patients with hypernatremia caused by loss of hypotonic fluid. Using the formula in the section on hyponatremia, one may calculate the estimated correction 1 L of any fluid will have on Na+. As with hyponatremia, hypernatremia with clinical manifestations warrants admission to an intensive care unit so that correction may be achieved safely and effectively.
Potassium: Potassium is the major intracellular cation. Plasma potassium ion (K+) concentration is determined primarily by two factors. The first is acid-base homeostasis. Hydrogen ion (H+) and K+ are exchanged between the intracellular and extracellular spaces, thus disturbances of acid-base balance (see below) tend to cause disturbances in serum K+. The second is the size of the total body K+ pool. Intracellular stores of K+ are large, but may be exhausted, especially in the setting of prolonged ketoacidosis.
Hypokalemia
Etiology: In the surgical setting, total body K+ is typically decreased by gastrointestinal losses, excessive diuretic administration, and prolonged malnutrition, particularly in alcoholic patients. Prolonged alkalosis (see below) that also results in hypokalemia (eg, due to gastric losses of hydrochloric acid and K+) results in a so-called “paradoxical aciduria” as the nephron conserves K+ at the expense of H+, which maintains the alkalosis instead of correcting it an attempt to prevent life-threatening hypokalemia (see below for details).
Presentation: The hallmark signs of hypokalemia are decreased muscle contractility eventually leading to diaphragmatic paralysis, EKG changes including a flattened or inverted T wave and a prominent U wave, and cardiac arrhythmias, which may present an immediate threat to life.
Treatment: When hypokalemia develops acutely it should be corrected with parenteral supplementation. Parenteral administration of K+ must be done carefully so as not to cause iatrogenic hyperkalemia. Hypomagnesemia may cause hypokalemia refractory to parenteral administration. In such cases hypomagnesemia should be corrected along with hypokalemia (see below). In cases of chronic hypokalemia in which neuromuscular and cardiac manifestations are absent, oral supplementation (either through dietary changes or oral potassium chloride administration) should suffice.
Hyperkalemia
Etiology: In the surgical setting, hyperkalemia is often caused by crush injuries, burns and other catabolism-inducing events, renal insufficiency, adrenal insufficiency, and excessive K+ administration. Acidosis may cause hyperkalemia as the intracellular space buffers acidemia by exchanging H+ from the extracellular space for K+ from within cells (see below).
Presentation: Hyperkalemia has few outward signs and symptoms until potentially lethal cardiac arrhythmias manifest. Initial EKG changes include flattened P waves and peaked T waves. Widening of the QRS complex is a later finding and demands immediate intervention, as it portends the imminent onset of ventricular fibrillation.
Treatment: A serum K+ of 6.5 mmol/L or greater is a medical emergency and must prompt immediate intervention. The patient must be placed on continuous EKG monitoring. Initial treatment should consist of intravenous administration of 50% dextrose in water, 10 units of regular insulin, and calcium gluconate, as well as inhaled β-adrenergic agonists like albuterol. Insulin, glucose, and β-agonists drive K+ from the extracellular space into the intracellular space, while calcium gluconate increases the excitability threshold of the myocardium, protecting against arrhythmias. If these measures are unsuccessful, hemodialysis may be required. Slowly developing hyperkalemia not severe enough to warrant intravenous interventions may be treated with oral sodium polystyrene sulfonate, which causes a slow enteral K+ wasting.
Magnesium: The magnesium ion (Mg2+) is an essential cofactor in many of the most important biochemical reactions in the body. Adenosine triphosphate (ATP) must be bound to Mg2+ to be biologically active. Mg2+ is required for every step of DNA transcription and translation, nerve conduction, ion transport and Ca2+ channel activity. Approximately 50%-60% of total body magnesium is found in the bones. The large majority of the rest is intracellular, and approximately 1% is extracellular. A constant proportion of dietary Mg2+ is absorbed by the gut. If gut absorption exceeds Mg2+ needs, the excess is excreted by the kidneys. If dietary intake is insufficient the kidney retains Mg2+, with urinary levels dropping to nearly zero.