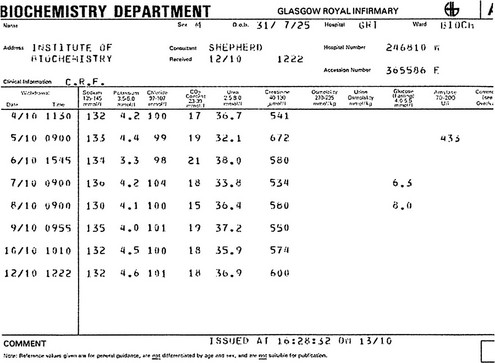
6 Fluid and electrolyte balance is central to the management of any patient who is seriously ill. Measurement of serum sodium, potassium, urea and creatinine, frequently with bicarbonate, is the most commonly requested biochemical profile and yields a great deal of information about a patient’s fluid and electrolyte status and renal function. A typical report is shown in Figure 6.1. Fig 6.1 A cumulative report form showing electrolyte results in a patient with chronic renal failure. A schematic way of representing fluid balance is a water tank model that has a partition and an inlet and outlet (Fig 6.2). The inlet supply represents fluids taken orally or by intravenous infusion, while the outlet is normally the urinary tract. Insensible loss can be thought of as surface evaporation. The water tank model illustrates the relative volumes of each of these compartments and can be used to help visualize some of the clinical disorders of fluid and electrolyte balance. It is important to realize that the assessment of the volume of body fluid compartments is not the undertaking of the biochemistry laboratory. The patient’s state of hydration, i.e. the volume of the body fluid compartments, is assessed on clinical grounds. The term ‘dehydration’ simply means that fluid loss has occurred from body compartments. Overhydration occurs when fluid accumulates in body compartments. Figure 6.3 illustrates dehydration and overhydration by reference to the water tank model. When interpreting electrolyte results it may be useful to construct this ‘biochemist’s picture’ to visualize what is wrong with the patient’s fluid balance and what needs to be done to correct it. The principal features of disordered hydration are shown in Table 6.1. Clinical assessments of skin turgor, eyeball tension and the mucous membranes are not always reliable. Ageing affects skin elasticity and the oral mucous membranes may appear dry in patients breathing through their mouths. Table 6.1 The principal clinical features of severe hydration disorders
Fluid and electrolyte balance
Concepts and vocabulary
Body fluid compartments
Feature
Dehydration
Overhydration ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree

 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access