Pretest self-assessment question (answer at the end of the case)
A 54-year-old patient has depression with prominent cognitive symptoms and also has the Val/Val genotype for catechol-O-methyltransferase (COMT). Based only on this genetic result, what treatment might be preferred for this patient?
A. SSRI
B. SNRI
C. NDRI
 Patient evaluation on intake
Patient evaluation on intake
54-year-old man was admitted to the hospital for an MDE
Experienced his first MDE at age 30; since then has had periodic MDEs of two-to-three months duration almost every fall/winter
 Psychiatric history
Psychiatric history
At age 37 had first inpatient psychiatric admission
Admitted again as an inpatient at ages 43 and 45
However, received no psychopharmacologic treatment other than sporadic St. John’s Wort for any of these episodes; he seemed to respond to this treatment plan
At age 47 was again hospitalized with an MDE
– Characterized by depressed mood, psychomotor retardation, cognitive impairment, reduced drive, sleep problems, delusions of guilt, and suicidal thoughts
 Social and personal history
Social and personal history
Smokes cigarettes regularly, does not drink or use illicit drugs
Is single and does not have any children
 Medical history
Medical history
There are no current medical problems
 Family history
Family history
He does not have any significant family history of psychiatric disorder
 Medication history
Medication history
At this admission to the inpatient unit, he was treated with prescription psychotropic medications for the first time
Experienced slight improvement but continued to have reduced drive, concentration deficits, psychomotor retardation, and suicidal thoughts
He developed EPS with risperidone treatment and was switched to another atypical antipsychotic, quetiapine (Seroquel) 400 mg/d, still with only partial MDD improvement
Mirtazapine (Remeron) was then switched to the SSRI sertraline (Zoloft) 200 mg/d and experienced some additional improvement in his mood but not in his concentration or fatigue
Next, received a series of 18 ECT sessions, while continuing only sertraline (Zoloft)
He obtained full remission status post-ECT and was discharged, with maintenance ECT and continuation of his SSRI recommended
Upon ECT service discharge, he was sent to outpatient psychiatry clinic for follow-up care
 Patient evaluation on initial visit
Patient evaluation on initial visit
Presents now, at age 54, with depressed mood, severe lack of drive, concentration deficits, memory problems, slow thinking, extreme fatigue, rigid facial expressions and gestures, and suicidal thoughts
His symptoms could be characterized in part as “psychiatric parkinsonism” with lack of drive and problems with concentration and memory, psychomotor retardation, slower thinking (bradyphrenia), and problems with facial expression and emotional gestures
– These Parkinson’s-like symptoms are caused by his MDD
He is not currently taking any medications as he stopped sertraline (Zoloft) after inpatient care but before his visit to outpatient department
 Question
Question
Based on this patient’s history and current symptom profile, testing of which of the following genes might be useful?
SLC6A4 (SERT)
SLC6A4 and COMT
SLC6A4, COMT, and methylenetetrahydrafolate reductase (MTHFR)
SLC6A4, COMT, MTHFR, and calcium channel voltage-dependent L-type, alpha-1c subunit (CACNA1C)
SLC6A4, COMT, MTHFR, CACNA1C, and D2 receptor (DRD2)
 Attending physician’s mental notes: initial evaluation
Attending physician’s mental notes: initial evaluation
Testing of any of these genes may provide information that could be considered in the management of this patient
– SLC6A4, 5HTTLPR Long(L)/Short(S) promoter insertion/deletion (rs63749047) and L(A)/L(G) (rs25531) polymorphism
This patient is homozygous (i.e., has two copies) for L(A)/L(A)
May indicate individuals who are more likely to exhibit response to SSRI treatment (compared to those with the S or L(G) allele)
They have more normal SERT function
They are hypothetically more resilient to stress-induced triggers for depression and suicide
L(A)/L(A) alleles are considered good*
– COMT, 158 Val>Met (472 G>A, rs4680)
This patient is homozygous for (158 Val/Val, 472 G/G)
May indicate individuals with depression who are more likely to experience associated cognitive symptoms such as slowness of information processing, difficulty with executive functioning, and problem solving
They have greater reductions in DA levels in prefrontal cortices
VAL/VAL alleles are considered bad
This patient is homozygous for T/T
May indicate individuals with depression who are also more likely to experience associated cognitive symptoms, especially in those who also express the Val variant of the COMT gene
These patients utilize brain L-methylfolate inefficiently, thus lowering their ability to synthesis serotonin, NE, and DA
T/T alleles are considered bad
– CACNA1C, G>A rs1006737
This patient is homozygous for (G/G)
The A allele (not carried by this patient) may indicate individuals with mood disorders who are more likely to experience frequent relapses and recurrences
They may lack neuronal membrane ion channel stability and allow excessive activity perhaps in limbic areas
G/G alleles are considered good
– DRD2, -141C insertion/deletion (rs1799732)
This patient is homozygous for (Ins/Ins)
May indicate individuals who are more likely to benefit from augmentation with an atypical antipsychotic in the event that they do not respond to an antidepressant (compared to those who carry the Del allele)
These patients hypothetically have more normalized function of the D2 receptors in associated neuronal pathways
Ins/Ins alleles are considered good
*For simplicity and teaching purposes, the alleles in this case are labeled as good or bad. These are not basic science nor clinical terms. Instead, these terms are used to get the reader accustomed to alleles being protective against psychiatric symptoms and disorders or increasing one’s risk of developing symptoms or disorders. Good means protective in general and bad means that risks increase. Additionally, the reader should be advised that it takes many alleles of many different genes interacting, and all of these interactions must work together with the environment (psychosocial stressors) in order for psychiatric symptoms to occur. For sake of simplicity, it is easier to start with good and bad to learn about the genetic underpinnings of psychiatric symptoms and how to bridge the gap between basic science and clinical application.
 Case outcome: initial visit
Case outcome: initial visit
No psychotherapy is offered
No prescription is issued
In considering the potential future of psychopharmacology, the patient has his saliva or cheek swab analyzed and is tested for risk genes for MDD
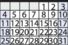
His results came back as listed in Table 28.1
| Pathway | Gene | Protein | Result |
|---|---|---|---|
| Serotonin | SLC6A4 | SERT, also called serotonin reuptake pump, responsible for termination of serotonin action | L(A)/L(A) |
| Dopamine | DRD2 | D2 receptor, target of antipsychotic drugs, theoretically overactive in psychosis and underactive in Parkinson’s disease | (Ins/Ins) |
| COMT | Enzyme responsible for degradation of DA and NE | (158 Val/Val, 472 G/G) | |
| Glutamate | CACNA1C | Voltage-gated channel for calcium | (G/G) |
| Metabolism | MTHFR | Predominant enzyme that converts inactive folic acid to active folate | (T/T) |
 Question
Question
Based on this patient’s symptoms, history, and genetic testing results, which of the following would you prescribe?
 Attending physician’s mental notes: initial evaluation (continued)
Attending physician’s mental notes: initial evaluation (continued)
Carrying both the COMT 158 Val/Val and the MTHFR 677 T/T genotype theoretically could result in increased degradation of DA in the prefrontal cortex, leading to decreased DA signaling there and associated cognitive dysfunction
Theoretically, it is plausible that the effect of the COMT Val allele on DA neurotransmission, which is further enhanced by the epistatic genetic interaction with the MTHFR T allele, could be a central explanation for the severe cognitive impairments of this patient, particularly with regard to his executive functions (“prefrontal dopamine” hypothesis)
– This patient cannot synthesize as much DA for later use (MTHFR T allele)
– This patient breaks down DA at a higher rate (COMT 158 Val/Val allele)
– The net effect is less globally available DA in the CNS
In addition, these genotypes might also be a good theoretical explanation for the “psychiatric parkinsonism” symptoms: lack of drive and concentration, memory disorder, psychomotor retardation, slower thinking (bradyphrenia) and movement (bradykinesia)
– The development of EPS in this patient on a rather low dose of risperidone (3 mg/d) is another sign of low DA function
– The patient’s genetic test results suggest that although he could respond to a serotonergic antidepressant (despite having failed monotherapy), a prodopaminergic drug might best address his symptoms as his “psychiatric parkinsonism” features might reflect poor dopaminergic clinical functioning
– Prodopaminergic options include NDRI, MAOI, and possibly augmentation with a stimulant or wakefulness-promoting agent
The patient’s genetic test results might also suggest that he should not receive augmentation with an antipsychotic, as these agents are DA antagonists and he may not tolerate these very well, and thus potentially exhibit further EPS or cognitive decline
To compensate the decreased capacity to convert folic acid to methylfolate (MTHFR: 677 T/T), L-methylfolate (Deplin) might be a beneficial augmentation strategy
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


