and Aysegul A. Sahin2
(1)
Division of Pathology, Singapore General Hospital, Singapore, Singapore
(2)
The University of Texas, M. D. Anderson Cancer Center, Houston, TX, USA
Keywords
FibroepithelialBreastFibroadenomaPhyllodesGradeRecurrenceMetastasisFibroepithelial neoplasms of the breast are characterised by proliferation of both epithelial and stromal components and include the common fibroadenoma and the less frequently occurring phyllodes tumour.
Fibroadenoma
Definition
The fibroadenoma is a common benign fibroepithelial tumour of females in the reproductive age group, although it may be diagnosed at any age.
Clinical and Epidemiological Features
Fibroadenomas present clinically as single or multiple lumps or nodules which are mobile, smooth contoured, and untethered to the skin on palpation.
Imaging Features
Mammographic screening has led to detection of fibroadenomas in mature women, often observed radiologically as typically well-defined, wider-than-tall nodules that may have gentle lobulations. Characteristic coarse “popcorn” calcifications are common (Fig. 3.1). There may be internal septations, which appear echogenic on sonography and as non-enhancing thin lines on magnetic resonance imaging.


Fig. 3.1
Radiology of fibroadenoma. (a) In a 42 year old woman with a right axillary palpable lump, the mammogram shows a well-defined, gently lobulated, oval nodule in the right axilla (white arrow). Accessory breast tissue is also seen (red arrow). (b) Ultrasound shows a well-defined oval nodule in the right axilla which was confirmed to be a fibroadenoma on core biopsy. The hyperechoic tissue surrounding the nodule is related to accessory breast tissue. (c) In this craniocaudal (CC) mammogram view of a right breast, there are two lobulated ‘popcorn’ calcifications that are classical for involuting calcified fibroadenomas (Courtesy of Dr. Lester Leong)
Pathologic Features
Macroscopic Pathology
Macroscopically, the fibroadenoma is usually encapsulated and shows circumscribed borders with a whitish-grey, bulging cut surface in which slit-like spaces are often evident (Figs. 3.2, 3.3, 3.4, 3.5, and 3.6). It is often rubbery in consistency and may have lobulated outlines. They can achieve large sizes, with fibroadenomas exceeding 5 cm in dimension being referred to as giant fibroadenomas.

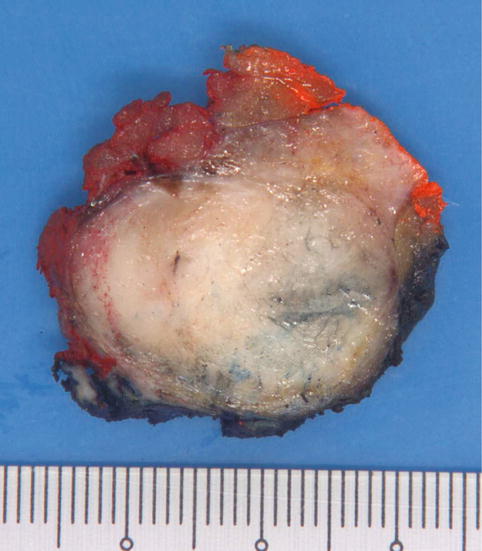




Fig. 3.2
Gross appearance of a fibroadenoma with a whitish whorled cut surface. The lobulated contours are seen as broad-based, bulging protrusions

Fig. 3.3
Macroscopy of a fibroadenoma with myxoid areas, seen towards the periphery of the lesion

Fig. 3.4
Giant fibroadenoma measures 5.5 cm in maximum dimension, showing multiple lobulations with smooth external surfaces

Fig. 3.5
Infarcted fibroadenoma shows extensive areas of haemorrhage. Its circumscribed outline delineates it from the surrounding fibrous breast tissue

Fig. 3.6
A juvenile fibroadenoma shows a circumscribed border with a whitish fibrous cut surface containing slit-like spaces (Courtesy of Dr. Kenneth Chang)
Microscopic Pathology
Histologically, the fibroadenoma shows two patterns: “intracanalicular”, with stroma compressing epithelial elements, and “pericanalicular”, with stroma growing around open ductules (Figs. 3.7, 3.8, and 3.9). These microscopic patterns do not have clinical significance and often occur together in the same tumour. The epithelial compartment may show any alterations that can be observed in the breast, from usual ductal hyperplasia (Fig. 3.10), columnar cell change, atypical hyperplasia (Fig. 3.11), and in situ (Fig. 3.12) and invasive carcinomas (Fig. 3.13). It is reported that atypical hyperplasia confined within the fibroadenoma does not incur an increased risk of subsequent breast cancer development [1].








Fig. 3.7
Fibroadenoma. At scanning magnification, the lesion shows circumscribed and partly encapsulated margins, with proliferation of both epithelial and stromal components

Fig. 3.8
Intracanalicular growth pattern of a fibroadenoma shows stroma growing into the epithelial compartment, compressing the epithelium, and stretching the ducts into elongated arc-like formations. The epithelium is bilayered, with luminal and myoepithelial layers

Fig. 3.9
Fibroadenoma with a predominantly pericanalicular growth pattern with the stroma growing around patent tubules

Fig. 3.10
Fibroadenoma with usual ductal hyperplasia. In this fibroadenoma, there is moderate usual ductal hyperplasia, with proliferation of epithelial cells forming protrusions into the lumen as well as displaying slit-like spaces among the epithelial cells

Fig. 3.11
Fibroadenoma with lobular neoplasia. (a) The epithelial compartment of this fibroadenoma shows distension of the acinar spaces by a proliferation of epithelial cells. Higher magnification shows the epithelial elements being filled by a discohesive population of rounded uniform epithelial cells with open vesicular nuclei, indistinct nucleoli, and thin cytoplasmic rims (inset) (b) Immunohistochemistry for E-cadherin shows absent staining in the lobular neoplastic cells. Cells that show positive reactivity are myoepithelial (seen as a peripheral rim) as well as residual luminal epithelial cells that are largely obscured by the lobular neoplastic cells

Fig. 3.12
Fibroadenoma with ductal carcinoma in situ (DCIS), low nuclear grade, cribriform pattern, without necrosis. (a) The circumscribed border of the fibroadenoma separates DCIS occurring within the lesion from the surrounding breast tissue. The predominantly intracanalicular growth pattern of the fibroadenoma with hyalinised stroma is seen in the lower right field. (b) Cribriform structures formed by uniform epithelial cells occupying the epithelial compartment of the fibroadenoma. (c) Immunohistochemistry for CK14 shows diminished staining within the cribriform epithelial population, with patchy albeit attenuated staining of myoepithelial cells at the periphery of ducts affected by DCIS. (d) Immunohistochemistry with ER shows diffuse nuclear staining of epithelial cells forming the cribriform structures

Fig. 3.13
Invasive lobular carcinoma (classic) in a fibroadenoma. (a) Core biopsy of a radiologic density in a 49-year-old woman. The architecture of a fibroadenoma is present at low magnification, showing an intracanalicular growth pattern. Part of the core shows a more cellular infiltrate occupying the stroma. (b) Higher magnification shows streams of cells with small nuclei encircling the epithelial compartment of the fibroadenoma, which is similarly involved by a monotonous cell population. (c) The epithelial compartment of the fibroadenoma is slightly distended by a similar population of monotonous cells of lobular carcinoma in situ, with nuclear features resembling those of the invasive component. (d) The invasive lobular carcinoma cells permeate in linear cords and as dispersed neoplastic cells within the hyalinised stroma of the fibroadenoma
The stroma can exhibit multinucleated giant cells (Fig. 3.14), which do not indicate stromal atypia. Fibroadenomas with cellular stroma are referred to as cellular fibroadenomas (Fig. 3.15), though the level of stromal cellularity needed for this diagnosis is subjective and may differ among pathologists. Fibroadenomas in children and young females under 20 years of age tend to have increased stromal cellularity and need to be distinguished from phyllodes tumours [2]. The presence of extensive myxoid stroma is seen in the myxoid fibroadenoma (Fig. 3.16). While myxoid fibroadenomas are described in the Carney’s complex (myxoid neoplasms and pigmented skin lesions), most observed in the breast are unassociated with this clinical condition. In older women, the stroma can become hyalinised and sclerotic. Calcifications within the stroma can lead to radiological detection (Fig. 3.17). Lipomatous, smooth muscle (Fig. 3.18), and osteochondroid metaplasia may be seen (Fig. 3.19). Pseudoangiomatous stromal hyperplasia can be present (Fig. 3.20). Stromal mitotic activity is usually scarce, though small numbers of stromal cell mitoses may be seen in fibroadenomas in young or pregnant patients. In paediatric patients, mitotic activity can range up to seven mitoses per ten high-power fields [3]. Infarction may occur during pregnancy (Figs. 3.21).









Fig. 3.14
Fibroadenoma with stromal multinucleated giant cells. (a) At low magnification, the stroma shows scattered cells with hyperchromatic nuclei. (b) The stromal multinucleated cells show smudged multilobated nuclei without discernible nucleoli or mitoses. These stromal cells do not contribute to assessment of stromal atypia in fibroepithelial lesions. (c) The stromal multinucleated cells can demonstrate a wreath-like arrangement of nuclei with inconspicuous pale cytoplasm. Adjacent stromal cells do not disclose significant nuclear atypia nor mitotic activity

Fig. 3.15
(a) Low magnification view of a cellular fibroadenoma, with relatively uniform stromal cellularity punctuated by benign epithelial elements. (b) Cellular fibroadenoma shows increased stromal cellularity, with plump stromal cells surrounding tubules in a pericanalicular growth pattern

Fig. 3.16
Myxoid fibroadenoma. Epithelial elements are seen within a myxoid stroma containing greyish-blue ground substance

Fig. 3.17
Hyalinised calcified fibroadenoma. These lesions are detected on screening mammography and may be radiologically indeterminate leading to core biopsy for evaluation. This core biopsy shows scant epithelial elements among a hyalinised stroma. A fractured calcification is present in the lower left field

Fig. 3.18
Fibroadenoma with smooth muscle metaplasia in the stroma. (a) Upper field shows a low magnification view of the fibroadenoma with an intracanalicular growth pattern. The stroma discloses vague bundles of plump spindle cells with pink cytoplasm. The lower field shows high magnification of the spindle cells with elongated nuclei and tapered cytoplasm. (b) Immunohistochemistry for smooth muscle actin shows positive staining bundles in the stroma, with high magnification delineating the spindle cells confirming their smooth muscle nature

Fig. 3.19
Ossified fibroadenoma. Metaplastic bone formation is seen in the stroma of the core biopsy of this fibroadenoma. Inset shows bone deposition around the epithelial elements of this hyalinised fibroadenoma

Fig. 3.20
Fibroadenoma with pseudoangiomatous stromal hyperplasia. The stroma of the fibroadenoma shows slit-like spaces lined by spindle nuclei. This may give the impression of increased stromal cellularity especially when there are scattered chronic inflammatory cells as well

Fig. 3.21
Infarcted fibroadenoma. (a) At low magnification, the infarcted fibroadenoma shows areas of haemorrhage. (b) Areas of haemorrhage are seen in the stroma, with ghost outlines of necrotic epithelial and stromal cells
The juvenile fibroadenoma is encountered in younger patients, though it has been documented in older adults as well (Figs. 3.22 and 3.23). Often demonstrating a cellular stroma, the term juvenile fibroadenoma has sometimes been used synonymously with cellular fibroadenoma. We prefer to diagnose a juvenile fibroadenoma when there is a predominant pericanalicular growth pattern accompanied by usual ductal hyperplasia that may resemble epithelial changes of gynaecomastia (gynaecomastoid hyperplasia), in addition to cellular stroma featuring intersecting fibroblastic and myofibroblastic fascicles. Sometimes the epithelial changes mimic atypical ductal hyperplasia.



Fig. 3.22
Juvenile fibroadenoma in a 26-year-old woman. (a) Circumscribed contours are seen at low magnification. (b) The juvenile fibroadenoma shows well-delineated borders and a high density of epithelial elements. A few adjacent rounded nubbins of lesional tissue are present. (c) The epithelial component of the juvenile fibroadenoma shows a pericanalicular pattern with moderate usual ductal hyperplasia giving rise to sieve-like formations, embedded within a fairly cellular spindled stroma. The pattern of usual ductal hyperplasia with micropapillary and cribriform appearances may mimic atypical ductal hyperplasia. (d) The spindled stromal cells comprising both fibroblasts and myofibroblasts are disposed in sweeping fascicles, swirling around the epithelial structures. Stromal spindle cell nuclei are bland, elongated, and occasionally compressed. (e) The epithelial cells show columnar change with apical snouts, with a few short tufts peeping into the lumen, linking up to form bridges encircling irregular spaces

Fig. 3.23
Juvenile fibroadenoma in a paediatric patient. (a) Low magnification shows epithelial elements recapitulating the terminal ductal lobular unit dispersed within a slightly cellular stroma. (b) Medium magnification shows usual ductal hyperplasia with occasional pink viscid secretions within the duct lumens. The stroma shows relatively even and diffusely increased cellularity. (c) Ductal epithelial hyperplasia here is mostly mild, with short micropapillary and snoutlike protrusions into the luminal spaces. Surrounding stromal cells show ovoid-to-elongated plump vesicular nuclei (Courtesy of Dr. Kenneth Chang)
The complex fibroadenoma is diagnosed when there is sclerosing adenosis, epithelial calcifications, papillary apocrine metaplasia, and cysts measuring 3 mm or more in size [4]. The presence of any of these features can designate a fibroadenoma as “complex”, though in our practice, we often reserve this diagnosis for fibroadenomas that either display a marked degree of these alterations or combination of alterations (Figs. 3.24 and 3.25). According to one study, complex fibroadenomas are associated with an increased relative risk of subsequent breast cancer (threefold), but the available data are insufficient to justify a different management approach for these lesions.




Fig. 3.24
Complex fibroadenoma. (a) Mammograms show a rounded opacity (arrow) in the left breast near the nipple (craniocaudal view). (b) Ultrasound shows a well-defined mass with internal echoes and cysts, measuring up to 2.6 cm


Fig. 3.25
Complex fibroadenoma. (a) Scanning view shows a well-circumscribed nodule punctuated by multiple cysts, expanding against the surrounding rim of breast tissue. (b) Cysts are lined by apocrine epithelium with papillary hyperplasia. (c) Higher magnification of the cysts lined by apocrine cells with luminal protrusions. (d) Sclerosing adenosis within a complex fibroadenoma, demonstrating closely packed tubules, some of which are compressed by myoepithelial cuffs. At low magnification, sclerosing adenosis appears to have a vaguely rounded contour. (e) Higher magnification of sclerosing adenosis shows both compressed as well as patent bilayered tubules. (f) Several patent lumens show pink secretions, with some appearing condensed and hardened into calcifications. (g) High magnification of the tubules containing luminal pink secretions, some appearing thick, hardened and almost calcific
Differential Diagnosis
The rare myxoma, and the even rarer low-grade fibromyxoid sarcoma, may mimic the myxoid fibroadenoma, especially when the epithelial component is scant (Fig. 3.26).


Fig. 3.26
(a) Low-grade fibromyxoid sarcoma, presenting as an axillary mass on screening mammography. At scanning magnification, the tumour has circumscribed boundaries with a spindle cell proliferation amid oedematous myxoid background. A cursory view may raise the differential of a myxoid fibroadenoma, except that this tumour is completely devoid of participating epithelial elements. (b) Higher magnification shows relatively bland spindle cells with variably myxoid and more cellular zones. The diagnosis was confirmed on fluorescence in situ hybridisation with the presence of FUS break-apart
The tubular adenoma consists of closely packed, rounded bilayered tubules resembling those in resting lobules. It is often encapsulated. Intervening stroma is usually scant and may appear cellular with accompanying lymphocytes. Grossly circumscribed, it can resemble the fibroadenoma, although it tends to be yellowish to tan brown with a softer consistency (Fig. 3.27). Histologically, it differs from the fibroadenoma by the dense arrangement of rounded tubules and the relative paucity of stroma. Luminal pink colloid-like secretions may be observed within the tubular lumens (Fig. 3.28).



Fig. 3.27
Tubular adenoma. On gross inspection, the tubular adenoma appears greyish brown and is of softer consistency. Occasional curvaceous slit-like spaces are seen. A rim of adjacent adipose breast tissue is noted around the upper contour of the lesion

Fig. 3.28
Tubular adenoma. (a) At low magnification, the tubular adenoma shows a densely packed collection of rounded tubules with scant intervening stroma, contrasting with the fibroadenoma in which there is accompanying stromal proliferation separating the epithelial elements. Note the circumscribed boundary with adjacent breast tissue, with a semblance of a thin capsule in parts. (b) At higher magnification, the tubules show bilayering with cuboidal to columnar luminal epithelium rimmed by a variably conspicuous myoepithelial layer that may demonstrate clear-to-pale cytoplasm. Luminal epithelial cells may disclose vesicular nuclei with visible nucleoli that can sometimes be potentially worrying when scrutinized at high magnification. The absence of significant nuclear pleomorphism or mitoses, together with the well-preserved myoepithelial layer, are reassuring features. Note the scant stroma in between the tubules, with some tubules almost touching one another. In the pericanalicular fibroadenoma, the tubules are more dispersed and widely separated by stroma
Adenosis is defined by the presence of an increased number of acinar/ductular units, with retention of the overall lobular architecture on low-power histological assessment (Fig. 3.29). It may resemble the fibroadenoma and tubular adenoma when it forms an expansile nodular lesion, but is more often a microscopic process that lacks encapsulation. Nodular sclerosing adenosis is a closely related entity that comprises compressed tubules with a pseudoinvasive pattern, mimicking invasive lesions like tubular carcinoma.


Fig. 3.29
Nodular adenosis. There is a nodular expansion of the lobule by an increased number of acini/ductules, some of which show mild dilatation. The periphery of this adenotic focus is rounded and pushing. There can be overlapping features with the tubular adenoma when the acini become closely packed, though nodular adenosis tends to be a microscopic alteration that generally does not lead to symptomatic presentation
The lactating adenoma occurs during pregnancy and lactation. While fibroadenomas and tubular adenomas with superimposed lactational changes may be referred to as lactating adenomas, most lactating adenomas tend to reflect lactational alterations in hyperplastic lobules. Grossly, it is circumscribed with brownish colouration and soft consistency (Fig. 3.30). Haemorrhage and infarction may be present (Fig. 3.31).



Fig. 3.30
Lactating adenoma. Macroscopic appearance of a lactating adenoma, sliced through to demonstrate its solid lobulated greyish-brown cut surface, disclosing intervening yellowish fatty areas as well as a few cysts

Fig. 3.31




Lactating adenoma. (a) At low magnification, the lactating adenoma shows enlarged lobules with increased numbers of closely packed acini. Some lobules disclose a greater degree of lactational change with dilated lumens, pink lining epithelium, and a few luminal epithelial projections. (b) Medium magnification shows juxtapositioning of a lobule with established lactational changes with one where secretory alterations are less well developed. Inset shows lining epithelial cells with pink bubbly cytoplasm with apical blebs and snouts
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


