Fig. 45.1 Endocrine control of the menstrual cycle.
FSH drives the developing ovarian follicle to convert androgens produced by its thecal cells into oestradiol (Fig. 44.2) within its granulosa cells. Oestradiol secretion from the follicle slowly rises as the follicle matures. In the window of time between the early to mid-follicular phase of the menstrual cycle the modest amount of oestrogen secreted by the follicle exerts negative feedback on both the hypothalamus and pituitary to keep gonadotropin secretion low (Fig. 43.1). The low plasma concentration of progesterone also weakly suppresses gonadotropin secretion in the early follicular phase.
In the late follicular phase a cohort of granulosa cells in the maturing ovarian follicle differentiates under the influence of FSH and starts to express LH receptors. These granulosa cells can then be stimulated by LH to secrete progesterone and are destined to become the corpus luteum after ovulation. In the mid- to late follicular phase the circulating oestradiol concentration rises dramatically as the follicle secretes more of the hormone under the influence of FSH. Eventually the plasma oestradiol reaches a critical concentration of about 200 pg · mL−1 for 48 h. The rapid rise and sustained high concentration of oestradiol triggers a switch from negative feedback to positive feedback of oestradiol upon the pituitary and hypothalamus, causing pulsatile release of GnRH. As a result there is an acute mid-cycle surge of LH for about 48 h, which is essential for ovulation. Ovulation involves release of the secondary oocyte from the follicle, which rapidly matures into an ovum.
Following ovulation (about day 14 of the menstrual cycle) the residual follicle transforms into the corpus luteum under the influence of the pituitary gonadotropins. The plasma LH concentration falls rapidly and remains low throughout the secretory (luteal) phase (days 15–26 of the menstrual cycle). The reason for this is that granulosa cells expressing LH receptors proliferate in the corpus luteum and produce increasing amounts of progesterone. Progesterone suppresses LH and FSH production by negative feedback on the hypothalamus and pituitary. If implantation of a fertilised ovum does not occur the corpus luteum regresses after about 10 days, since it requires gonadotropins to maintain itself.
From day 5 to late in the menstrual cycle the gradually increasing plasma concentrations of oestrogens, and subsequently progesterone, which are produced as the menstrual cycle progresses, result in proliferation and vascularisation of endometrial cells that are able to secrete a variety of fluids and nutrients aimed at making the endometrium receptive for implantation. The temporal precision of the change in receptivity is critical if successful implantation of a fertilised oocyte is to occur. Oestrogen and progesterone cause the endometrium to become oedematous, and its glands secrete increasing quantities of amino acids, sugars and glycoproteins in a viscous liquid. At the end of the menstrual cycle the circulating concentrations of progesterone and oestrogen eventually fall to levels that no longer support the endometrium. Deprived of hormonal support, the endometrial spiral arteries go into spasm and the endometrial cells die, producing digestive enzymes (ischaemic phase: days 27–28 of the menstrual cycle). As a consequence of this and other changes the endometrium is shed during menstruation.
The cervical mucus is also influenced by oestrogen and progesterone concentrations. Under the dominant influence of progesterone cervical mucus is viscid and less penetrable by sperm, whereas at ovulation the high plasma oestradiol concentration results in thinner and more elastic mucus that is easily penetrable by sperm. Progesterone also inhibits the motility of the fallopian tube, altering the transport of sperm, and the fertilised or unfertilised oocyte. Excess progesterone may alter the chance of fertilisation occurring or the embryo may reach the uterine cavity when the endometrium is not receptive to implantation. Oestrogens have the opposite action, increasing tubal motility, and may accelerate the transport of the ovum into the uterine cavity.
Physiology of pregnancy
Pregnancy is accompanied by considerable hormonal changes. When a fertilised ovum implants in the uterine lining the corpus luteum becomes essential for the production of progesterone and maintenance of pregnancy during the first 6–8 weeks. After this placental production of hormones takes over and the combined feto-placental unit produces progressively greater quantities of oestrogen and progesterone which reach the maternal circulation. The dividing cells of the implanted ovum start to secrete human chorionic gonadotropin (HCG) from about 9 days after fertilisation (Ch. 43). HCG stimulates the corpus luteum to continue to secrete progesterone. Eventually, the placenta takes over the production of HCG, progesterone and oestrogen. The placenta also produces human placental lactogen as pregnancy advances, resulting in the development of duct and milk-secreting cells in the mother’s breasts. The precise balance of sex steroids also contributes to quiescence of the uterus during pregnancy and the onset of labour at term.
Mechanism of action of oestrogens and progestogens
In common with other steroid hormones, both oestrogens and progestogens act by influencing gene transcription (see also Ch. 44). They passively diffuse into the cell and bind to specific receptors in either the cytoplasm or cell nucleus (see Fig. 1.8). The receptors are associated with heat-shock protein (HSP) when in their unbound state in the cytosol. HSP dissociates when the hormone binds to the receptor, and the receptor forms dimers that are translocated by active transport to the cell nucleus. The steroid–receptor complex associates with hormone-response elements of numerous oestrogen- or progesterone-responsive genes. This leads to recruitment of co-activator molecules to the complex, and produces gene transcription (co-activation). Oestrogen binds to two specific cytoplasmic receptors (ERα and ERβ), which have different tissue distributions. Progesterone also has two specific receptors (PR-A and PR-B) that regulate progesterone-responsive genes. Oestrogen increases and progesterone decreases the expression of the progesterone receptors.
Steroidal contraceptives
Oral hormonal contraceptives (‘the pill’) are the most widely used form of contraception and contain either a combination of a synthetic oestrogen with a synthetic progestogen (a C19 synthetic progesterone derivative) or a progestogen alone.
Mechanisms of hormonal contraception
Elevated circulating concentrations of synthetic oestrogen and progestogen prevent the precise cyclic pattern of hormone-related events seen in the normal menstrual cycle (Fig. 45.2), and can be used for contraception.
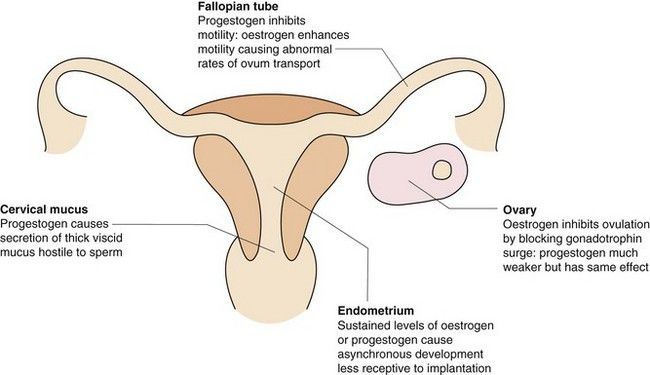
Fig. 45.2 The main contraceptive actions of the synthetic oestrogens and progestogens in the combined hormonal contraceptive pill.
FSH, follicle-stimulating hormone; LH, luteinizing hormone.
 The combination of oestrogen and progestogen exerts its contraceptive effect mainly through suppression of FSH release, which prevents development of the follicles in the ovary. The lack of a dominant follicle means that production of oestradiol is impaired. The failure of plasma oestradiol to rise, combined with negative feedback from the progestogen, prevents the mid-cycle LH surge that is essential for ovulation to occur.
The combination of oestrogen and progestogen exerts its contraceptive effect mainly through suppression of FSH release, which prevents development of the follicles in the ovary. The lack of a dominant follicle means that production of oestradiol is impaired. The failure of plasma oestradiol to rise, combined with negative feedback from the progestogen, prevents the mid-cycle LH surge that is essential for ovulation to occur. Progestogen produces asynchronous development of the endometrium with stromal thinning, which makes it less receptive to implantation of the fertilised ovum. Fallopian tube motility is increased by oestrogens and decreased by progestogens; this may affect fertility by altering the rate of transport of the ovum.
Progestogen produces asynchronous development of the endometrium with stromal thinning, which makes it less receptive to implantation of the fertilised ovum. Fallopian tube motility is increased by oestrogens and decreased by progestogens; this may affect fertility by altering the rate of transport of the ovum.Progestogens can be used alone for contraception, when the mechanism depends on the dose of progestogen. Low-dose progestogen inhibits ovulation in only about 50% of cycles, and contraception relies upon the other actions of the hormone. With higher doses, inhibition of follicular development and ovulation becomes more important.
The ‘combined’ hormonal contraceptive
Combined hormonal contraceptives (often called the combined oral contraceptive pill, or COCP) contain both a synthetic oestrogen and progestogen. The oestrogen component is usually ethinylestradiol (an oestrogen that is alkylated at C17 to slow its metabolism) but in some combinations is mestranol, a compound that is metabolised in the liver to ethinylestradiol. Over the years since the combined oral hormonal contraceptive was introduced, the dose of the oestrogen component has been reduced to minimise unwanted effects. ‘Second-generation’ combined hormonal contraceptives have a lower oestrogen concentration than ‘first-generation’ combined hormonal contraceptives, which are no longer used. The lowest dose of oestrogen that gives good menstrual cycle control (absence of breakthrough bleeding; see below) is preferred.
The progestogen component of the second-generation combined oral hormonal contraceptives is either levonorgestrel (the active isomer of norgestrel) or norethisterone; these compounds are testosterone analogues that also possess residual androgenic activity. ‘Third-generation’ oral combined hormonal contraceptives contain modified progestogens that have less androgenic activity – desogestrel, gestodene, norgestimate (which are all derivatives of norgestrel) – or anti-androgenic activity – dienogest, drospirenone (a derivative of the aldosterone antagonist spironolactone with some anti-mineralocorticoid activity). Modified progestogens are used if there are unacceptable unwanted effects with the second-generation progestogens.
Other differences and similarities between second- and third-generation combined hormonal contraceptives are discussed below.
Monophasic preparations
Monophasic preparations contain fixed amounts of oestrogen and progestogen. They are taken daily for the first 21 days of the menstrual cycle followed by seven contraceptive-free days with tablets containing an inactive substance, such as lactose. The oestrogen concentration should be the lowest that maintains good cycle control and produces minimal unwanted effects. There is a choice of:
The monophasic oral combined hormonal contraceptive contains one of several progestogens. In some women it may be necessary to change the formulation to reduce minor unwanted effects, such as breakthrough bleeding or weight gain during the menstrual cycle. The degree of androgenic activity possessed by different progestogens (see combined hormonal contraceptive section above) may influence the suitability of an individual preparation for a particular woman.
A transdermal patch formulation of low-strength ethinylestradiol with the third-generation progestogen norelgestromin is also available; this is applied weekly for 3 weeks followed by a 7-day patch-free interval. There is also a vaginal contraceptive ring that contains ethinylestradiol with the progestogen etonorgestrel.
Biphasic and triphasic preparations
Biphasic and triphasic preparations are designed to mimic more closely the changes in sex hormone concentrations that occur during the natural menstrual cycle. The total sex hormone intake through the cycle is no less than with monophasic preparations. Several preparations are available, all of which contain ethinylestradiol in combination with levonorgestrel, norethisterone or gestodene. The dose of ethinylestradiol is either kept constant throughout, as in the monophasic pills, or increased during days 7–12. Progestogen doses are increased once (biphasic) or twice (triphasic) as the menstrual cycle proceeds.
Progestogen-only contraceptives
Oral progestogen-only contraceptives
The oral progestogen-only contraceptive (‘progestogen-only pill’, or POP) is particularly useful for women in whom the administration of oestrogen is considered to be undesirable, for example if there is a history of thromboembolic disorders (see below). Pregnancy rates are slightly higher than with a low-dose combined oral hormonal contraceptive. Various progestogens are used, such as desogestrel, etynodiol diacetate, gestodene, levonorgestrel or norethisterone. The progestogen-only contraceptive must be taken daily, without a break, and within 3 h of the usual time every day (see efficacy below). Because the dose of progestogen is low, bleeding does occur at monthly intervals but may be irregular. Breakthrough bleeding occurs in up to 40% of women; this is much higher than with the combined hormonal contraceptive. Some women become amenorrhoeic while using progestogen-only contraception.
Parenteral progestogen-only contraceptives
Intramuscular injection of a progestogen, either medroxyprogesterone acetate or norethisterone, can provide contraception for up to 8–12 weeks. The higher dose of progestogen compared with the oral preparations reliably inhibits ovulation, and therefore there is a low incidence of ectopic pregnancy. The contraceptive effect is fully reversible, but there is a high incidence of amenorrhoea when its effect wears off. Prolonged use of medroxyprogesterone acetate can reduce bone mineral density and cause osteoporosis. The loss of bone mineral density occurs over the first 2–3 years of use and then stabilises. Prolonged use of medroxyprogesterone acetate beyond 2 years, or its use in adolescents or people with other risk factors for osteoporosis, is discouraged.
A subcutaneous implant of etonogestrel provides contraception for up to 3 years, after which time it should be replaced. The progestogen is released from a flexible rod inserted subdermally on the lower surface of the upper arm. Local irritation is experienced by some women. The implants are radio-opaque so they can be easily located by radiography. Unwanted effects are similar to those experienced with the oral progestogen-only contraceptive, but lower doses of progestogen are needed because first-pass metabolism in the gut and liver is avoided.
Intra-uterine progestogen-only device
A plastic intra-uterine contraceptive system (IUS) with a levonorgestrel-releasing system from a silicone reservoir provides effective contraception with reduced menstrual blood loss compared with copper intra-uterine contraceptive devices (IUCDs) that do not contain a progestogen, and carries less risk of pelvic inflammatory disease. The progestogen is released from the device for a period of 5 years. The device is also used to control menstrual bleeding in women with primary menorrhagia by preventing endometrial proliferation.
Efficacy of hormonal contraception
When taken according to the recommended schedule, the failure rate for the combined hormonal contraceptive is 0.2%. With the combined oral hormonal preparations, contraceptive protection is reduced if there is a delay of more than 24 h in taking the daily dose. In such circumstances the missed dose should be taken as soon as possible. If two doses are missed then additional contraceptive measures should be used for 7 days.
Failure of the progestogen-only oral contraceptive is age-related and is up to 5% in young women, falling with decreasing fertility to about 0.3% at the age of 40 years. With the oral progestogen-only contraceptive, other contraceptive precautions should be taken for 2 days if there is a delay of only 3 h or more after the normal time of taking the daily dose.
Emergency contraception
This can be carried out with the progestogen levonorgestrel, the progesterone receptor modulator ulipristal acetate, or a copper-containing IUCD. A single large dose of levonorgestrel is taken within 72 h after unprotected intercourse, and preferably within 12 h. Levonorgestrel inhibits ovulation, but only if taken before the LH surge. The treatment is successful in up to 99% of cases, but the efficacy is greatly reduced if used between 72 and 120 h after unprotected intercourse. Nausea is a frequent unwanted effect, occurring in up to 22% of women, and an anti-emetic (e.g. domperidone; Ch. 32) may be needed. Absorption takes 2 h and vomiting after this time will not affect the efficacy of treatment. A larger dose may be required if drugs that induce drug-metabolising enzymes in the liver are being taken. In the UK, levonorgestrel can be purchased without prescription by women over the age of 16 years.
The progesterone receptor modulator ulipristal acetate suppresses the mature follicle up to and including the time of the LH surge, so can be effective when taken up to 120 h after unprotected intercourse. Insertion of a copper IUCD up to 5 days after unprotected sexual intercourse is more effective as emergency contraception than levonorgestrel, but it is not known whether it is more effective than ulipristal acetate.
Pharmacokinetics of contraceptive steroids
The synthetic oestrogens, like the naturally occurring oestradiol-17β (oestradiol), and progestogens are highly lipid-soluble molecules that are rapidly and completely absorbed from the gut lumen after oral administration. Synthetic drugs are metabolised more slowly, including less first-pass metabolism, than the natural hormones oestradiol (half-life 1–2 h) and progesterone (half-life 5–20 min), so they have greater oral bioavailability and longer half-lives. For example, ethinylestradiol undergoes some first-pass metabolism (about 20%) but this is low compared with oestradiol (90–95%), and its half-life is longer (8–24 h). Some synthetic drugs are prodrugs that undergo first-pass metabolism to the active entity. Oestrogens and progestogens are eliminated by hepatic metabolism, often involving CYP3A4-mediated oxidation and/or conjugation with glucuronic acid and/or sulphate. The conjugates may undergo enterohepatic cycling. Enterohepatic cycling of ethinylestradiol is responsible for maintaining effective plasma concentrations with low-dose formulations. There is considerable inter-individual variation in plasma levels of oestrogens and progestogens after ingestion of the combined hormonal contraceptive.
The kinetics of oestrogens and progestogens can be affected by the administration of other drugs. Contraceptive failure may occur if there is concomitant treatment with drugs that induce liver cytochrome P450 enzymes (Table 2.7), such as anticonvulsants (e.g. barbiturates, carbamazepine or phenytoin), antiretroviral drugs (e.g. nelfinavir, nevirapine or ritonavir) or antibacterials (e.g. rifampicin and rifabutin) (Ch. 51). A higher dose of ethinylestradiol (using multiple tablets) should be used during and for 4 weeks after stopping these drugs. Alternatively, a form of contraception unaffected by enzyme-inducing drugs should be used (such as an IUCD or parenteral progestogen).
The pharmacokinetics of individual synthetic oestrogens and progestogens vary widely and details are given in the drug compendium at the end of this chapter.
Beneficial and unwanted effects of contraceptive steroids
 Cancer: there is a 40% reduction in the risk of ovarian cancer after 5 years of use and persisting for up to 15 years after stopping. Endometrial cancer is reduced by 50%, with a similar duration of protection.
Cancer: there is a 40% reduction in the risk of ovarian cancer after 5 years of use and persisting for up to 15 years after stopping. Endometrial cancer is reduced by 50%, with a similar duration of protection. Acne can be treated with combined oral hormonal contraceptives, since they reduce the concentration of free testosterone (Ch. 49). A combination of ethinylestradiol with cyproterone acetate, a weak progestogen with antiandrogenic activity (Ch. 46), is sometimes used for this purpose.
Acne can be treated with combined oral hormonal contraceptives, since they reduce the concentration of free testosterone (Ch. 49). A combination of ethinylestradiol with cyproterone acetate, a weak progestogen with antiandrogenic activity (Ch. 46), is sometimes used for this purpose.Unwanted effects
Both oestrogens and progestogens have a number of minor and major unwanted effects, but the incidence of the major effects, although important, is relatively low.
 Thromboembolism: the incidence of venous thromboembolic disease is increased in some subgroups of women taking the combined hormonal contraceptive. The mechanisms are complex but include procoagulant activity from increased production of clotting factors X and II and decreased production of anticoagulant anti-thrombin (Ch. 11). Fibrinolysis is impaired, while reduced prostacyclin generation enhances platelet aggregation (Ch. 11). The risk of thromboembolism increases with age, and is greater in women who smoke (because smoking increases the risk of thrombogenesis) or who are obese and in those with a thrombophilic tendency, such as deficiency of protein C or protein S or the presence of factor V Leiden. The baseline risk of venous thromboembolism in women of reproductive age not taking the combined oral hormonal contraceptive is about 5 per 100 000 per year. The risk in women taking second-generation preparations (containing levonorgestrel) is about 15 per 100 000 per year and in those taking third-generation preparations containing desogestrel or gestodene (and possibly drospirenone) is about 25 per 100 000 per year. It is important that these risks are put into context; for example the risk of venous thromboembolism in pregnancy is 60 per 100 000 pregnancies.
Thromboembolism: the incidence of venous thromboembolic disease is increased in some subgroups of women taking the combined hormonal contraceptive. The mechanisms are complex but include procoagulant activity from increased production of clotting factors X and II and decreased production of anticoagulant anti-thrombin (Ch. 11). Fibrinolysis is impaired, while reduced prostacyclin generation enhances platelet aggregation (Ch. 11). The risk of thromboembolism increases with age, and is greater in women who smoke (because smoking increases the risk of thrombogenesis) or who are obese and in those with a thrombophilic tendency, such as deficiency of protein C or protein S or the presence of factor V Leiden. The baseline risk of venous thromboembolism in women of reproductive age not taking the combined oral hormonal contraceptive is about 5 per 100 000 per year. The risk in women taking second-generation preparations (containing levonorgestrel) is about 15 per 100 000 per year and in those taking third-generation preparations containing desogestrel or gestodene (and possibly drospirenone) is about 25 per 100 000 per year. It is important that these risks are put into context; for example the risk of venous thromboembolism in pregnancy is 60 per 100 000 pregnancies. Ischaemic heart disease and ischaemic stroke: there is an increased risk of myocardial infarction and stroke in women taking the combined hormonal contraceptive who smoke or who are hypertensive, particularly in those over the age of 35 years. The added risk in those over 40 years is 20 per 100 000 for smokers and 29 per 100 000 for women with hypertension. It has been suggested that enhanced thrombogenesis rather than premature atherogenesis is responsible for the excess cardiovascular risk with the combined hormonal contraceptive. The lowest possible dose of oestrogen should be given to older women who use the combined hormonal contraceptive.
Ischaemic heart disease and ischaemic stroke: there is an increased risk of myocardial infarction and stroke in women taking the combined hormonal contraceptive who smoke or who are hypertensive, particularly in those over the age of 35 years. The added risk in those over 40 years is 20 per 100 000 for smokers and 29 per 100 000 for women with hypertension. It has been suggested that enhanced thrombogenesis rather than premature atherogenesis is responsible for the excess cardiovascular risk with the combined hormonal contraceptive. The lowest possible dose of oestrogen should be given to older women who use the combined hormonal contraceptive. Increase in blood pressure: a small increase in blood pressure, typically 5/3 mmHg, is common during use of the combined hormonal contraceptive, but not of progestogen-only contraceptives. A significant rise can occur in about 5% of women with previously normal blood pressure and in up to 15% of women with pre-existing hypertension. The mechanism is probably an increase in plasma renin substrate (Ch. 6) produced by oestrogen and, to a lesser extent, progestogen. Blood pressure may remain elevated for some months after the combined hormonal contraceptive has been stopped. Regular monitoring of blood pressure is advisable during use of the combined hormonal contraceptive, and it should be stopped if the blood pressure rises above 160 mmHg systolic or 95 mmHg diastolic.
Increase in blood pressure: a small increase in blood pressure, typically 5/3 mmHg, is common during use of the combined hormonal contraceptive, but not of progestogen-only contraceptives. A significant rise can occur in about 5% of women with previously normal blood pressure and in up to 15% of women with pre-existing hypertension. The mechanism is probably an increase in plasma renin substrate (Ch. 6) produced by oestrogen and, to a lesser extent, progestogen. Blood pressure may remain elevated for some months after the combined hormonal contraceptive has been stopped. Regular monitoring of blood pressure is advisable during use of the combined hormonal contraceptive, and it should be stopped if the blood pressure rises above 160 mmHg systolic or 95 mmHg diastolic. Cancer: there is a small excess risk of breast cancer, but it is uncertain whether this relates to earlier diagnosis. The rate of diagnosis remains higher for 10 years after the combined hormonal contraceptive is stopped. The incidence of cervical cancer is slightly increased by combined hormonal contraceptives after 5 years of use.
Cancer: there is a small excess risk of breast cancer, but it is uncertain whether this relates to earlier diagnosis. The rate of diagnosis remains higher for 10 years after the combined hormonal contraceptive is stopped. The incidence of cervical cancer is slightly increased by combined hormonal contraceptives after 5 years of use. Nausea, mastalgia, depression, headache, weight gain and provocation of migraine may be minimised by prescribing preparations with low oestrogen content, or by changing the progestogen to desogestrel, drospirenone or gestodene. Women who have migraine with aura are at increased risk of stroke if they take a combined hormonal contraceptive.
Nausea, mastalgia, depression, headache, weight gain and provocation of migraine may be minimised by prescribing preparations with low oestrogen content, or by changing the progestogen to desogestrel, drospirenone or gestodene. Women who have migraine with aura are at increased risk of stroke if they take a combined hormonal contraceptive. Breakthrough bleeding occurs frequently in some women, whereas in others withdrawal bleeding fails to occur. Gestodene-containing pills or triphasic preparations probably give the best cycle control. Amenorrhoea after stopping the combined hormonal contraceptive can last beyond a few months in about 5% of women, and a small number can experience amenorrhoea for more than a year. A history of irregular periods before taking the combined hormonal contraceptive increases the chance of prolonged amenorrhoea.
Breakthrough bleeding occurs frequently in some women, whereas in others withdrawal bleeding fails to occur. Gestodene-containing pills or triphasic preparations probably give the best cycle control. Amenorrhoea after stopping the combined hormonal contraceptive can last beyond a few months in about 5% of women, and a small number can experience amenorrhoea for more than a year. A history of irregular periods before taking the combined hormonal contraceptive increases the chance of prolonged amenorrhoea. Metabolic effects: oestrogens alone increase protective plasma high-density lipoprotein (HDL) cholesterol, decrease low-density lipoprotein (LDL) cholesterol and increase plasma triglycerides (see also Ch. 48). When used in combination with the second-generation progestogens, HDL cholesterol is reduced. Oestrogens increase vascular prostacyclin and nitric oxide synthesis, inhibit platelet adhesion and suppress smooth muscle cell proliferation. Some progestogens such as norethisterone and medroxyprogesterone acetate may oppose the beneficial effects of oestrogens on the arterial wall. The third-generation combined hormonal contraceptives containing gestodene and desogestrel increase plasma triglycerides but, unlike the progestogens in the second-generation pills, they increase HDL cholesterol. The clinical relevance of these small changes is uncertain.
Metabolic effects: oestrogens alone increase protective plasma high-density lipoprotein (HDL) cholesterol, decrease low-density lipoprotein (LDL) cholesterol and increase plasma triglycerides (see also Ch. 48). When used in combination with the second-generation progestogens, HDL cholesterol is reduced. Oestrogens increase vascular prostacyclin and nitric oxide synthesis, inhibit platelet adhesion and suppress smooth muscle cell proliferation. Some progestogens such as norethisterone and medroxyprogesterone acetate may oppose the beneficial effects of oestrogens on the arterial wall. The third-generation combined hormonal contraceptives containing gestodene and desogestrel increase plasma triglycerides but, unlike the progestogens in the second-generation pills, they increase HDL cholesterol. The clinical relevance of these small changes is uncertain. Increased skin pigmentation can occur in some women who take oestrogens. The androgenic progestogens can sometimes cause or aggravate hirsutism and acne or produce weight gain. In women with hyperandrogenaemia (such as occurs with polycystic ovary syndrome) a third-generation combined hormonal contraceptive would be preferred, as gestodene and desogestrel have little androgenic activity.
Increased skin pigmentation can occur in some women who take oestrogens. The androgenic progestogens can sometimes cause or aggravate hirsutism and acne or produce weight gain. In women with hyperandrogenaemia (such as occurs with polycystic ovary syndrome) a third-generation combined hormonal contraceptive would be preferred, as gestodene and desogestrel have little androgenic activity. Effects on the liver are occasionally seen. Cholestatic jaundice can be produced by progestogens, and oestrogens increase the risk of gallstones.
Effects on the liver are occasionally seen. Cholestatic jaundice can be produced by progestogens, and oestrogens increase the risk of gallstones.Non-contraceptive uses of steroidal contraceptives
The combined hormonal contraceptive can be used:
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








