Chapter 3 Families of compounds that occur in essential oils
The chemistry of essential oils is organic and vast. To avoid confusion a formal system was developed: the IUPAC (International Union of Pure and Applied Chemistry) system. This names compounds based on the arrangement of the component atoms into functional groups, e.g. alcohols contain –OH.
There are two main types of component in essential oils: hydrocarbons (carbon and hydrogen only) and oxygenated hydrocarbons, which also contain oxygen. These are subdivided into groups based on their structures (Table 3.1). In this chapter, the general physicochemical and therapeutic properties associated with each group are given but it must be emphasized that not all members will have every property; for example acetic acid (vinegar) and the polyacids in dietary fat are all of the form X–COOH but differ drastically. Interactions with other groups in the molecule and in the oil can also affect properties.
Table 3.1 Two major classes of compounds found in essential oils
| Hydrocarbons | Oxygenated compounds |
|---|---|
| Terpenes based on the isoprene unit (5 carbon atoms) | Alcohols Phenols Aldehydes Ketones Ester Lactones |
| Monoterpenes: 2 isoprene units (up to 10 carbon atoms) | |
| Sesquiterpenes: 3 isoprene units (up to 15 carbon atoms) | |
| Diterpenes: 4 isoprene units (up to 20 carbon atoms) |
THE TERPENES
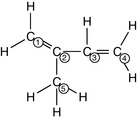
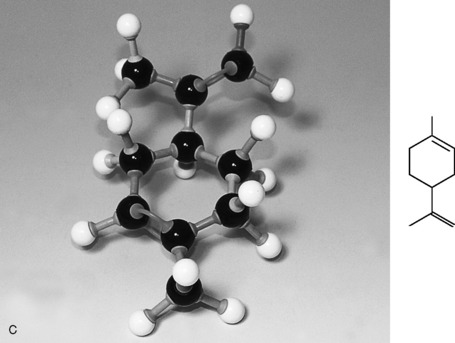
The terpenes are a large group of naturally occurring hydrocarbons (made up of carbon and hydrogen only) found in essential oils. They are based on the isoprene unit with the molecular formula C5H8 (Fig. 3.1). Isoprene is a chain structure described as aliphatic or acyclic, which means a compound with its carbon atoms in chains not closed rings:
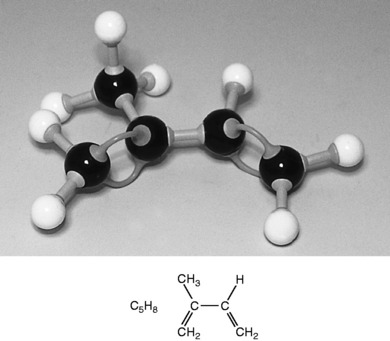
Figure 3.1 Isoprene (C5H8): The monomer, or single unit, that builds up into the terpenes.
Courtesy Spiring Enterprises Ltd.
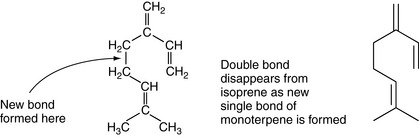
The isoprene unit acts as monomer or single unit that builds up in repeating units to make the groups of terpenes found in the essential oils. Their names usually end in -ene.
There are several groups of terpene hydrocarbon based on the number of isoprene units incorporated.
Monoterpenes
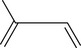
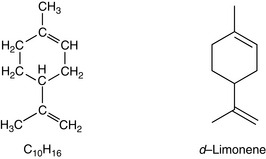
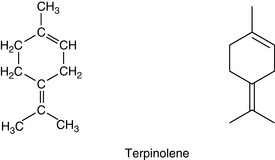
Monoterpenes are made up of two isoprene units, joined head to head. They have a molecular formula of C10H16 (Fig. 3.2).
Myrcene
Myrcene is an example of a monoterpene and is found in essential oils of bay, verbena, pine and juniper, and in many others.
Sesquiterpenes
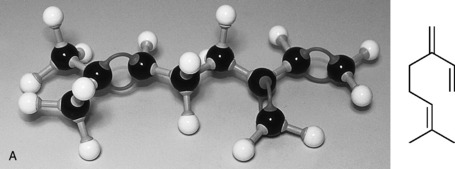
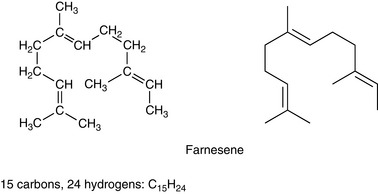
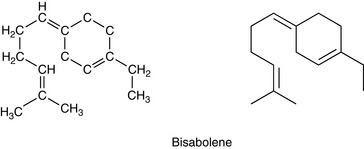
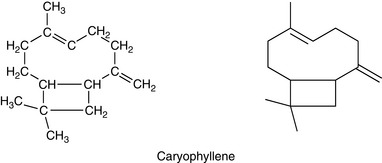
Sesqui means half as much again, so sesquiterpenes have a molecular formula one and a half times a monoterpene: they are made up of three isoprene units. The molecular formula is C15H24 (Fig. 3.3).
Bisabolene
A cyclic structure (with a carbon ring in the molecule) found in myrrh oil and German chamomile.
Caryophyllene
Cyclic and has a strong woody, spicy odour, found in oil cloves, lavender, sweet thyme and ylang ylang.
Diterpenes
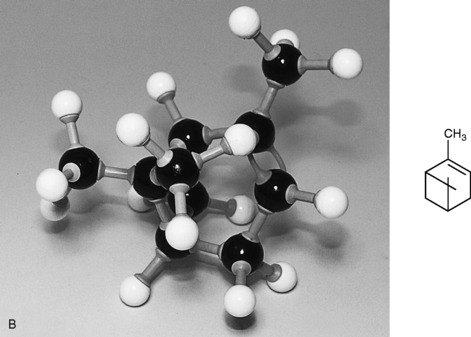
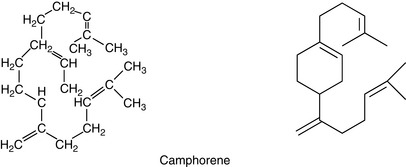
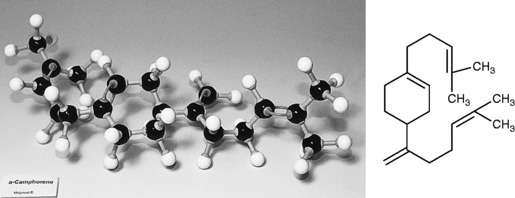
Camphorene
Cyclic compound, found in the high boiling fraction of camphor oil, boiling point (b.p.) 177–178 °C.
Properties of diterpenes
The molecular structure of the diterpene camphorene is given in Figure 3.4.
Physical and chemical properties
Diterpenes are similar to sesquiterpenes but the larger molecules and molecular weights give them higher boiling points and lower oxidation rates.
Polyterpenes
Polyterpenes are compounds comprising several hundred isoprene units and give rise to natural rubber. Poly means ‘many’, so that rubber is made up of many repeating isoprene units. The name for a compound made up of many such repeating units is a polymer. There are many both naturally occurring and synthetically produced polymers of importance with many applications.