Iris anomalies
Iridocorneal synechiae and other anomalies
Peter anomaly
Axenfeld anomaly
Rieger anomaly
Phacomatoses
Neurofibromatosis
Sturge–Weber syndrome
Rubella
Marfan syndrome
Persistent fetal vasculature
Retinopathy of prematurity
Retinoblastoma
Juvenile xanthogranuloma
Clinical
♦
Elevated intraocular pressure
♦
Corneal edema
♦
Generalized enlargement of the eye (buphthalmos)
Microscopic
♦
Degeneration and atrophy of inner layers of the retina, mainly the ganglion cell layer and optic nerve fiber layer
♦
Optic nerve atrophy with excavation (“cupping”) of the optic nerve head
♦
Corneal edema
♦
Rupture of the corneal Descemet membrane and Haab striae
♦
Atrophy of the iris and ciliary body
Acquired Glaucoma
Types
♦
Primary
Open-angle glaucoma or simple
Closed-angle glaucoma
♦
Secondary (see Table 25.2 and Fig. 25.1)
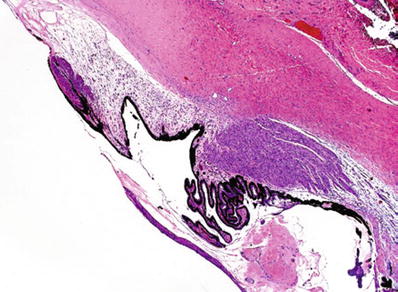
Table 25.2.
Conditions Associated with Secondary Glaucoma
Abnormalities of the native lens |
Lens dislocation |
Persistent flat anterior chamber (post eye trauma) |
Iridocorneal endothelial syndrome |
Anterior uveitis (iritis) |
Retinopathy of prematurity |
Persistent fetal vasculature |
Iris neovascularization |
Cysts of ciliary body and iris |
Juvenile xanthogranuloma |
Intraocular neoplasia |
Hemorrhage in anterior chamber |
Hemolysis |

Fig. 25.1.
Glaucoma. Closed-angle glaucoma due to extensive iridocorneal synechiae and obliteration of angle of anterior chamber in a patient with previous cataract surgery.
Microscopic
♦
Degeneration and atrophy of inner layers of the retina, mainly the ganglion cell layer and optic nerve fiber layer
♦
Optic nerve atrophy with excavation (“cupping”) of the optic nerve head
♦
Corneal edema
♦
Atrophy and fibrosis of the anterior uvea (iris and ciliary body)
Retinopathy of Prematurity (Retrolental Fibroplasia)
Clinical
♦
Develops in premature infants after birth
♦
Usually received supplemental oxygen therapy in early postnatal period
♦
May present with “white pupil” or “leukocoria”
♦
Often bilateral
Microscopic
♦
Capillary proliferation in the retina, extending into the vitreous
♦
Hemorrhages and fibrosis
♦
Fibrous membranes with tractional detachment of the retina
Fibrous mass behind the lens may simulate retinoblastoma
Persistent Fetal Vasculature (PFV) or Persistent Hyperplastic Primary Vitreous (PHPV)
Clinical
♦
Congenital anomaly present at birth
♦
Sometimes associated with other congenital anomalies
♦
Often associated with microphthalmia
♦
Usually unilateral
♦
May present with “white pupil” or “leukocoria”
Microscopic
♦
Loose fibrovascular tissue in the vitreous cavity (Fig. 25.2)
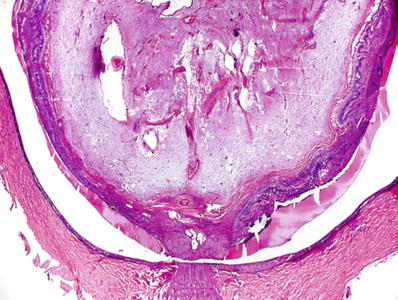

Fig. 25.2.
Persistent fetal vasculature. The vitreous cavity is occupied with a loose fibroconnective and adipose tissues and remnants of the hyaloid vessels. The vitreous contents are firmly adherent to the retina, which is detached and is dysplastic.
♦
Persistence of hyaloid vessels
♦
Adipose tissue, muscle fibers, and islands of cartilage
♦
Elongation of ciliary processes
♦
Retinal dysplasia
Inflammations of the Eye
♦
As in any other organ, inflammations may be acute or chronic
♦
May affect one or more coats of the eye or the transparent media
♦
Viruses, bacteria, fungi, and parasites may affect the eye (Fig. 25.3)
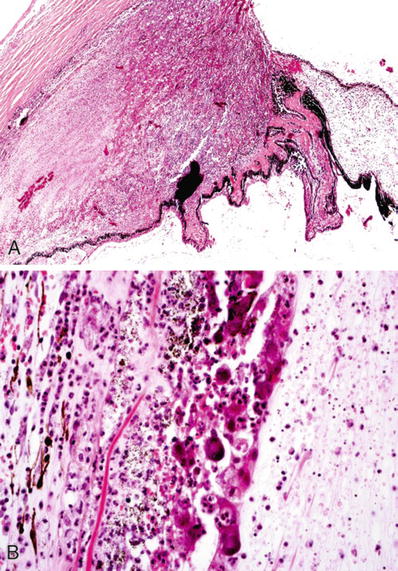

Fig. 25.3.
Viral infection. (A) Marked inflammation of the ciliary body due to CMV uveitis and retinitis in an HIV-positive patient. (B) CMV inclusions in necrotic retinal pigment epithelium.
♦
Infection occurs mainly by direct penetration of the infectious agent through a wound, traumatic or surgical
♦
Agents may reach the eye by extension of infectious processes from adjacent structures (i.e., paranasal sinuses) or hematogenous dissemination
Acute Inflammation
Endophthalmitis
Clinical
♦
Inflammation of the vitreous and inner coats of the eye
♦
Etiology: bacteria, fungi, and viruses (herpes virus, cytomegalovirus, measles)
Microscopic
♦
Suppurative inflammation in the anterior chamber and vitreous cavity (Fig. 25.4)
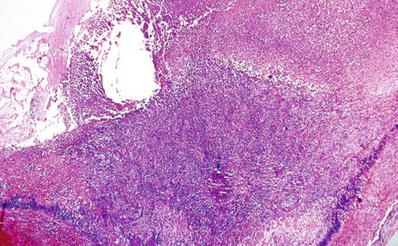

Fig. 25.4.
Acute suppurative endophthalmitis with inflammation and necrosis of all intraocular structure.
♦
Variable degrees of necrosis of the uvea and retina
♦
Tumors with extensive necrosis (melanoma and retinoblastoma) may present as endophthalmitis (Fig. 25.5)
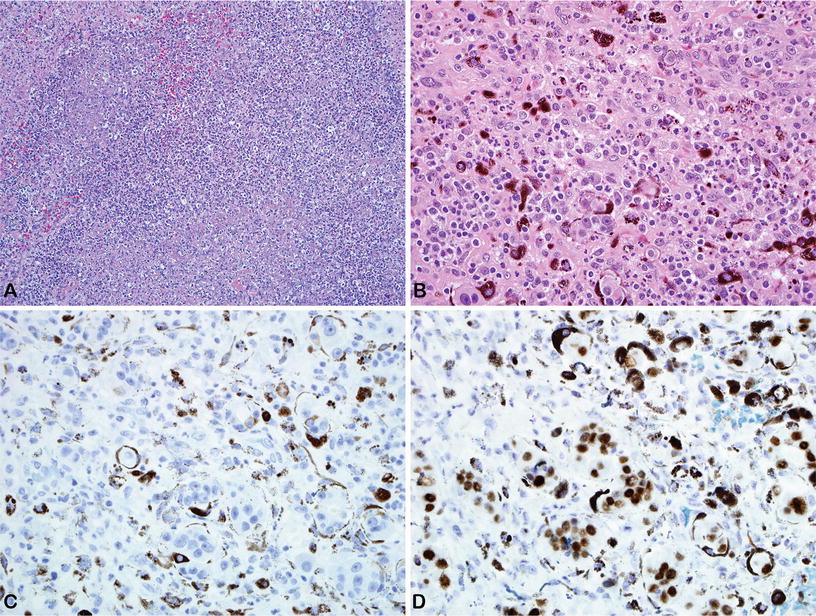

Fig. 25.5.
Patient had an evisceration because of severe endophthalmitis. Histological examination revealed a metastatic carcinoma of the breast with extensive necrosis. (A) Purulent-like necrotic tissue with severe mixed inflammatory cell infiltration. (B) Focus a viable metastatic tumor in the choroid. Immunohistochemical stains for (C) cytokeratin 7 and (D) GATA 3.
Panophthalmitis
Clinical
♦
Inflammation involving all coats of the eye, including the sclera
♦
Etiology is similar to endophthalmitis but viruses rarely affect the sclera
Microscopic
♦
Suppurative inflammation involving all chambers and coats of the eye
♦
Process may extend to adjacent orbital soft tissues (Fig. 25.6)
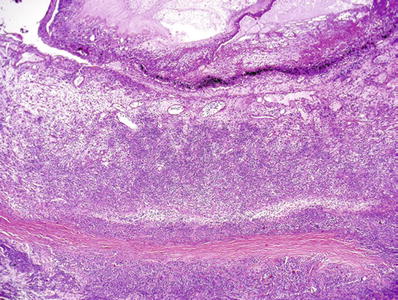

Fig. 25.6.
Acute suppurative panophthalmitis. Purulent exudates extend beyond the cavities of the eye with almost necrosis of the sclera.
Chronic Nongranulomatous Inflammation
Clinical
♦
Usually affects the uveal tract (uveitis)
♦
Etiology
Infectious agents
Noninfectious
Physical and chemical injury
Allergic
♦
Affects one or more uveal structures
Iritis
Cyclitis
Iridocyclitis
Choroiditis
♦
Uveitis may be associated with numerous syndromes
Ankylosing spondylitis
Reiter syndrome
Inflammatory bowel disease
Psoriasis
Microscopic
♦
Focal or diffuse small mononuclear cell infiltration
♦
Plasma cells may be the predominant elements in the infiltrate
♦
Iridocorneal synechiae, iris–lens synechiae, and glaucoma may follow repeated episodes of inflammation in the iris
♦
In the late stages of the disease, phthisis bulbi may develop
Chronic Granulomatous Inflammation
Sympathetic Uveitis (Sympathetic Ophthalmia)
Clinical
♦
Diffuse bilateral inflammation of the uveal tract following eye trauma or surgery
♦
Delayed T-cell-mediated autoimmune reaction to antigen or antigens released at the time of injury
♦
Develops 2 weeks to years after a penetrating or perforating trauma to the eye
♦
The contralateral eye (sympathizing eye) develops lesions similar to those present in the affected, previously injured eye (exciting eye)
♦
Uveal incarceration or prolapse into the wound is usually observed (Fig. 25.7)
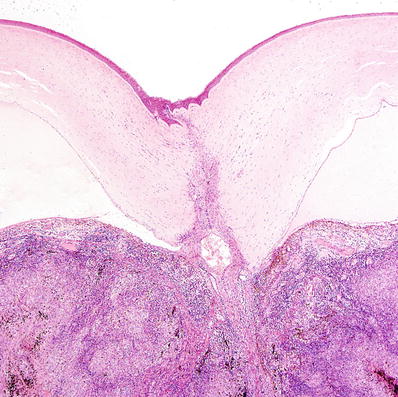

Fig. 25.7.
Perforating traumatic injury to the cornea with uveal incarceration and severe chronic granulomatous inflammation.
♦
Initial presentation characterized by blurred vision, photophobia, and mutton-fat keratic deposits
Microscopic
♦
Diffuse granulomatous inflammation involving the iris, ciliary body, and choroid (Fig. 25.8)
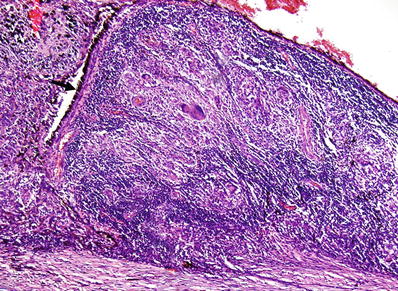

Fig. 25.8.
Sympathetic ophthalmia. Severe granulomatous inflammation in the choroid following penetrating eye trauma. The arrow points at the retinal pigment epithelium.
♦
The infiltrate is made up predominantly of epithelioid cells and lymphocytes
♦
The lymphocytic infiltrate includes almost exclusively T lymphocytes
♦
In the choroid, the choriocapillary layer is usually spared
♦
Epithelioid cells and multinucleated giant cells may contain phagocytized pigment granules in their cytoplasm
♦
Nodules of epithelioid cells and pigment-laden cells (Dalén–Fuchs nodules) in the subretinal space are characteristic but not pathognomonic (Fig. 25.9)
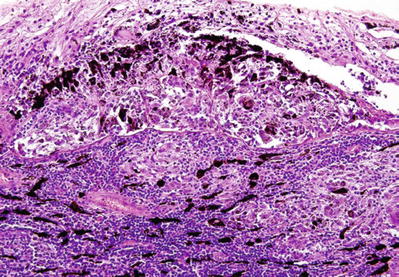

Fig. 25.9.
Sympathetic ophthalmia. Ill-defined granulomas with multinucleated giant cells and marked lymphoplasmacytic infiltration in choroid. Noted the large group of epithelioid cells and pigment epithelium in the subretinal space, the Dalén–Fuchs nodule, characteristic of sympathetic ophthalmia.
♦
Collections of macrophages on posterior surface of cornea (mutton-fat keratic deposits)
Phacoantigenic (Phacoimmune, Phacoanaphylactic) Endophthalmitis
Clinical
♦
Rare, severe granulomatous inflammation of the eye
♦
It is an immune complex disease with loss of immune tolerance
♦
Secondary to traumatic rupture of the lens capsule and release of lens proteins
♦
It may coexist with sympathetic ophthalmia
Microscopic
♦
Zonal granulomatous inflammation around lens with ruptured capsule (Fig. 25.10)
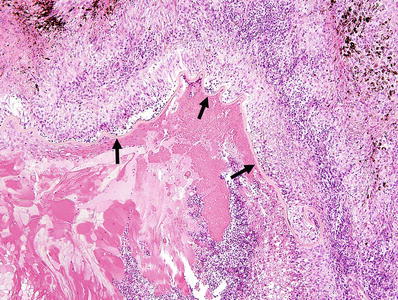

Fig. 25.10.
Phacoantigenic endophthalmitis following traumatic rupture of the lens. Note the “zonal” appearance of the inflammatory infiltrate around the wrinkled remnants of the lens capsule (arrows).
♦
First zone: prominent polymorphonuclear cell infiltration
♦
Second zone: layer of histiocytes and multinucleated giant cells
♦
Third zone: in the periphery, there is a layer of granulation tissue with lymphoplasmacytic infiltration
♦
Depending on the severity of the initial trauma, the uvea may also show nongranulomatous inflammation or sympathetic uveitis (ophthalmia)
Diabetes
Clinical
♦
Major cause of blindness in the USA and other Western countries
♦
Women are more commonly affected
♦
Diabetes and its complications may involve:
All coats and transparent media in the eye
Optic nerve and intracranial visual pathways
Ocular adnexa
Orbit
Microscopic
♦
Conjunctival microaneurysms
♦
Recurrent erosions and corneal ulcers (in patients with diabetic neuropathy)
♦
Neovascularization of the iris (rubeosis iridis)
♦
Cataracts
♦
Glaucoma
♦
Proliferative retinopathy with retinal detachments
♦
Retinal hemorrhages
♦
Ischemic optic neuropathy
Coat Disease
Clinical
♦
Exudative retinal detachment
♦
Usually unilateral
♦
Mainly affects males up to 18 years of age
♦
Clinically, it may mimic retinoblastoma
Microscopic
♦
Telangiectatic dilatation of retinal vessels, microaneurysms, and areas of ischemia
♦
Protein and lipid-rich transudate in outer retinal layers
♦
Subretinal exudates containing foamy macrophages and cholesterol crystals
♦
Retinal detachment
Massive Retinal Gliosis
Clinical
♦
Segmental or total replacement of neural retina by glial tissue
♦
Usually follows penetrating trauma to the eye
♦
Chronic inflammatory processes, congenital malformations, and retinal vascular disorders may also lead to massive retinal gliosis
Microscopic
♦
Large mass of glial cells that partially or totally fills the cavities of the eye
♦
Glial cells are elongated (piloid) and have long pale eosinophilic processes
♦
Rosenthal fibers and foci of calcification may occur within the mass (Fig. 25.11)
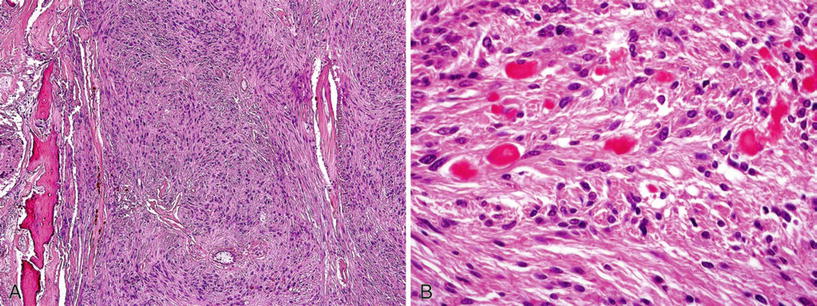

Fig. 25.11.
(A) Massive gliosis of the retina in an eye enucleated several years after severe ocular trauma followed by phthisis bulbi. Note the focus of ossification. (B) Rosenthal fibers in densely reactive glial proliferation.
♦
Blood vessels are usually dilated and have thin walls
♦
Degenerative changes in the intraocular tissue and foci of ossification may be observed
♦
Cells forming the mass are positive for glial fibrillary acidic protein (GFAP)
Phthisis Bulbi
Clinical
♦
End stage of many diffuse ocular diseases
♦
Usually submitted by the ophthalmologists with the diagnosis of “blind painful eye”
♦
Marked atrophy and shrinkage of the eyeball
Microscopic
♦
Marked shrinkage and disorganization of the eye (Fig. 25.12)
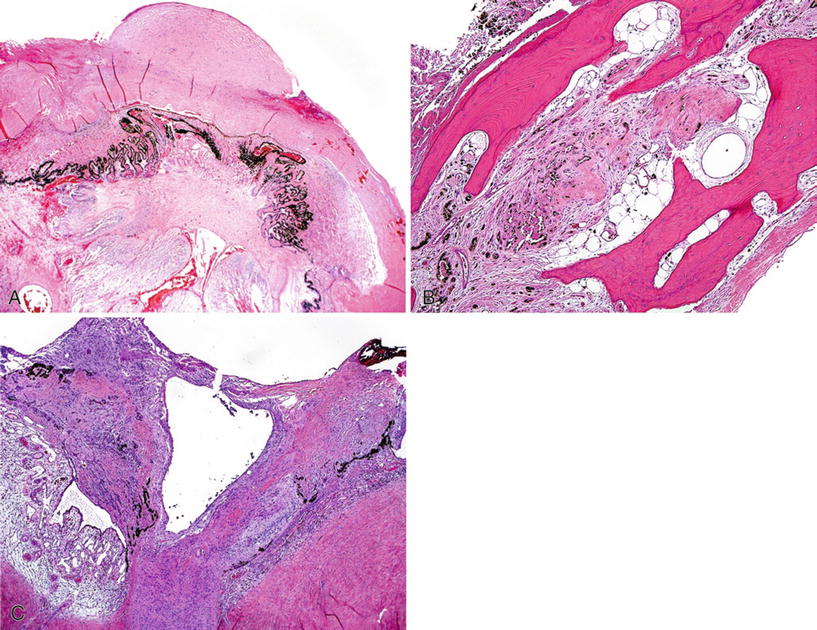

Fig. 25.12.
Phthisis bulbi. (A) Marked shrinkage of the entire globe with disorganization and atrophy of all intraocular structures. Note the scarring of the cornea, the atrophy and gliosis of the retina, and the extensive synechiae of the iris and ciliary body to the cornea. (B) Hyperplasia and metaplasia of the retinal pigment epithelium and foci of ossification. (C) Posterior aspect of the globe. Old retinal detachment with atrophy and gliosis of the retina and reactive proliferation of the retinal pigment epithelium.
♦
Corneal scarring with or without vascularization
♦
Thickened sclera
♦
Profound disorganization of intraocular tissues
♦
Foci of calcification and ossification are usually observed
♦
The osseous tissue may contain islands of the hematopoietic bone marrow
Cornea
Basic Anatomy of the Cornea
♦
The normal cornea is 11–13 mm in diameter
♦
It is 0.56 ± 0.03 mm thick in the center and 0.64 ± 0.03 mm in the periphery (may appear thicker after formalin fixation)
♦
The cornea has six anatomic layers:
Anterior corneal epithelium, five layers of nonkeratinizing squamous cells
Basement membrane of the anterior corneal epithelium
Bowman membrane: thick layer of collagen fibrils
Stroma represents 90% of the entire thickness of the cornea and is made up of multiple lamellae of collagen and fibroblasts (keratocytes)
Descemet membrane: thick basement membrane of the corneal endothelium
Corneal endothelium
♦
Blood vessels are not observed in the normal cornea
♦
There is a rich innervation (ophthalmic division of the trigeminal nerve)
♦
Mitoses are not observed in the anterior corneal epithelium except in the periphery (stem cell region). Cells are continuously replaced by sliding of cells from the periphery toward the center
Corneal Edema and Bullous Keratopathy
Clinical
♦
Corneal edema and bullous keratopathy are major indications for corneal transplantation or stripping of the Descemet membrane
♦
Many clinical conditions are associated with corneal edema
♦
It may occur sometime after intraocular surgery (i.e., cataract extraction)
♦
It is usually secondary to dysfunction of the corneal endothelium (“endothelial decompensation”)
♦
Edema may be bilateral but is often asymmetric
♦
Chronic corneal edema may result in the formation of bullae in the anterior corneal epithelium
♦
Rupture of the bullae is associated with severe pain and may lead to complications
♦
In the late stages, spindle cells from the stroma may migrate into the subepithelial space and form a dense fibrous layer (“fibrous pannus”)
Microscopic
♦
Increased fluid in the corneal stroma is difficult to document histologically because it causes separation of the lamellae (which is also a common artifact of histological processing)
♦
Edema of the anterior corneal epithelium is the most reliable diagnostic feature (Fig. 25.13A)
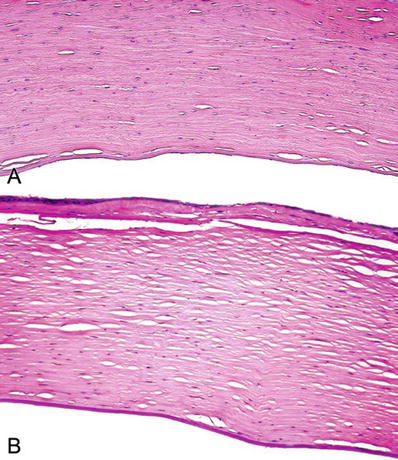

Fig. 25.13.
(A) The cornea. Edema in anterior corneal epithelium overlying a focus of posttraumatic stromal scarring. (B) Chronic corneal edema. Atrophy of anterior corneal epithelium and dense subepithelial fibrous pannus.
♦
The number of endothelial cells may be reduced
♦
Thickening and lamination of the Descemet membrane suggest dysfunction of the endothelial cells
♦
Wart-like protrusions on the posterior surface of the Descemet membrane (“guttae”) may develop. They are best demonstrated with the PAS stain
Stromal Scarring and Vascularization
♦
Scarring and vascularization of the corneal stroma are nonspecific
♦
They may occur as complication of trauma, inflammatory processes, ulcers, chemical burns, irradiation, and other conditions
♦
Scars may be focal or diffuse, depending on the cause of the injury
♦
The Bowman membrane and corneal stroma do not regenerate after an injury but are replaced by scar tissue (Fig. 25.14)
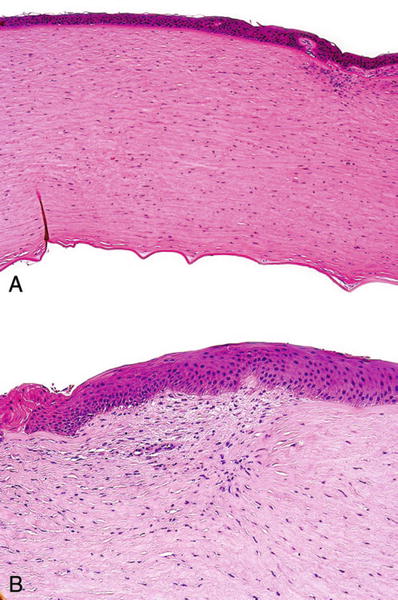

Fig. 25.14.
The cornea . (A) Scar in anterior corneal stroma following penetrating injury. (B) Scarring and vascularization of the anterior corneal stroma.
Blood Staining of the Cornea
Clinical
♦
Complication of hyphema (blood in the anterior chamber)
♦
Hemorrhage may be posttraumatic or spontaneous
♦
Usually, but not always, associated with high intraocular pressure
♦
Reddish discoloration of the cornea
Microscopic
♦
Red blood cells and breakdown products in the corneal stroma (Fig. 25.15)
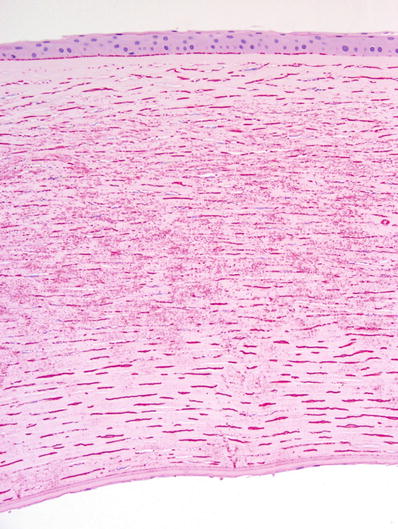

Fig. 25.15.
Blood staining of the cornea.
♦
Cytoplasm of keratocytes may contain hemosiderin
Failed Corneal Graft
Clinical
♦
Corneal transplants or penetrating keratoplasty is usually performed without providing any immunosuppression
♦
Success rate is markedly diminished if the cornea is vascularized
♦
Corneal graft failures are usually secondary to endothelial damage
Microscopic
♦
Changes are similar to those seen in chronic corneal edema and bullous keratopathy (Fig. 25.16)
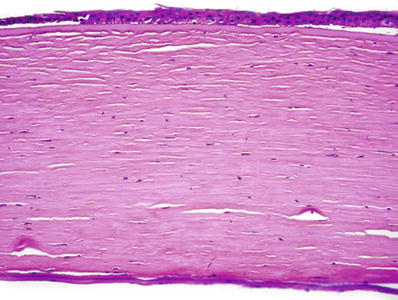

Fig. 25.16.
Failed corneal graft. Atrophy and partial disorganization of epithelial cells. The anterior corneal epithelium is partly detached from the underlying Bowman membrane. The inflammatory infiltration and vascularization of graft rejection are not observed.
♦
A layer of the fibrous tissue may develop on the posterior surface of the cornea (“retrocorneal fibrous plaque”). It usually starts in the area of the surgical wound
♦
Immune rejection of a corneal graft may occur. It causes marked vascular proliferation and inflammatory cell infiltration in the periphery of the corneal button
Corneal Opacification: Facette, Nebula, Macula, and Leukoma
♦
Facette
Small foci of thickening of the anterior corneal epithelium filling superficial defects with loss of the Bowman membrane and superficial corneal stroma
The normal curvature of the anterior surface of the cornea is preserved
♦
Nebula
Small delicate opacities in corneal stroma
Histologically, they of the anterior corneal epithelium filling superficial defects with loss of the Bowman membrane and superficial corneal stroma.The normal curvature of the aappear as small superficial scars
♦
Macula
Larger opacities due to more extensive scarring, usually deeper in the stroma
♦
Leukoma
Focus of scarring involving almost the entire thickness of the cornea stroma
Keratitis (Corneal Inflammations) and Corneal Ulcers
♦
Corneal erosions
Limited damage to the epithelium
Best seen clinically after fluorescein staining
♦
Keratitis
More extensive epithelial damage with variable depths of stromal involvement
Seen on macroscopic examination
Etiology
♦
Trauma
♦
Toxic
♦
Radiation induced
♦
Infectious
Viral
Adenovirus (several types, mainly type 8)
Herpes simplex virus
Herpes zoster virus
Epstein–Barr virus
Bacterial
Pyogenic bacteria
Syphilis
Trachoma
Lyme disease
Tuberculosis
Leprosy
Parasitic
Protozoa
◦
Amebiasis (Acanthamoeba)
◦
Leishmaniasis
◦
Trypa nosomiasis
Nematodes
◦
Onchocerciasis
♦
Other causes
Sarcoidosis
Lymphogranuloma venereum
Atopic keratoconjunctivitis
Lymphoproliferative disorders (mycosis fungoides, Hodgkin lymphoma)
Rosacea
Crohn disease
Syphilitic Keratitis
♦
Extensive stromal involvement
♦
Anterior uveitis present in early stages
♦
Congenital form
Usually bilateral
May be present at birth
Cloudy cornea due to inflammation and vascularization of deep stroma
It is part of the Hutchinson triad
♦
Acquired form
Late manifestation – 10 years after primary infection
Usually unilateral, sector shape lesion
Onchocerciasis
♦
The leading cause of blindness in the world, Africa and Central and South America (18 million children and young adults)
♦
Causes the river blindness
♦
Acute phase: nummular or snowflake corneal opacities
♦
Late phase: dense stromal scarring
♦
Microfilariae migrate through the skin and subcutaneous tissue
♦
Small, black fly, Simulium sp. transmits parasite from human to human
Peripheral Corneal Ulcers
Ring Ulcer and Ring Abscess
♦
Linear stromal ulcerated lesion that involves most of the entire circumference of the cornea
Usually follows accidental or surgical trauma
Starts at 1–2 mm from the limbus into the clear portion of the cornea
The rim of the clear cornea always remains
The central cornea rapidly undergoes necrosis
Panophthalmitis follows
The eye is lost
Usually infectious; collagen diseases may be the cause
Central Corneal Ulcers
Etiology
♦
Viral
♦
Ba cterial
♦
My cotic
♦
Parasitic
Herpes Simplex Keratitis
♦
The most common cause of central corneal ulcer
♦
Initially involves the epithelium and has a dendritic pattern
♦
Patients with atopic dermatitis – susceptible to dissemination
♦
The virus spreads from ganglion cells along the branches of the ophthalmic division of the trigeminal nerve
Bacterial Ulcers
♦
They are associated with severe inflammation of the corneal epithelium and stroma
♦
May contain a purulent exudate
Etiology
♦
Organisms that may cause corneal ulcers
Pneumococcus
Streptococcus
Pseudomonas
Klebsiella
Staphy lococcus
Other less virulent bacteria
Mycotic Ulcers
♦
Usually present with a “dry” main lesion and smaller satellite lesions
♦
Hypopyon (purulent exudates in anterior chamber) is common (Fig. 25.17)
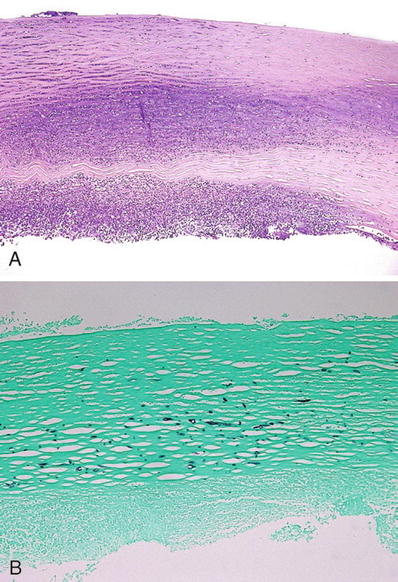

Fig. 25.17.
(A) Corneal ulcer with suppurative inflammation in the anterior chamber (hypopyon) due to fungal infection (Fusarium sp.). (B) Fungal organism in the stroma (GMS stain).
♦
Fungus may be isolated by scraping margins and depth of ulcer
♦
Agents isolated from ulcers may be in mycelial form (i.e., Aspergillus) or yeasts (i.e., Candida)
♦
Generally, they are secondary to trauma and contamination with plant or animal matter
Acanthamoeba Corneal Ulcer
♦
The agent is a free-living protozoon found in soil, freshwater, and human oral cavity
♦
Most cases occur in patients who wear contact lenses
♦
The lesion is usually a painful, central, or paracentral ulcer
♦
The inflammatory infiltrate predominates in the anterior and midsection of the stroma
♦
The process has a waxing and waning course
♦
An associated scleral infection may occur
♦
The diagnosis is confirmed by identification of the cysts in H&E-stained sections. Cyst walls stain with PAS, GMS, and Giemsa (Fig. 25.18)
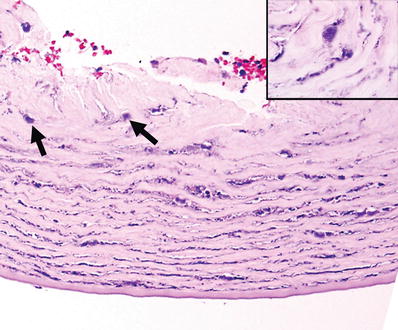

Fig. 25.18.
Acanthamoeba keratitis. Biopsy of a corneal ulcer with numerous encysted microorganisms (arrows). Inset: cyst of Acanthamoeba (oil immersion).
♦
Trophozoites are better identified in cultures
Complications of Corneal Ulcers
♦
Vascularization of the corneal stroma
♦
Scarring of the stroma may be focal or extensive and may involve only part or the entire thickness of the cornea
♦
Descemetocele is a herniation of the Descemet membrane through a defect of the corneal stroma secondary of a deep penetrating ulcer
♦
Ectasia, partial or diffuse protrusion of a thin cornea secondary to the inflammatory process
♦
Staphyloma. Lesion is a corneal ectasia with a bluish color produced by the pigmented uveal tissue behind the cornea
♦
Xerosis. Dryness and secondary keratinization of the cornea due to the destruction of conjunctival goblet cells and lacrimal glands by the inflammatory process
Corneal Degenerations and Dystrophies
Band Keratopathy (Calcific Band Keratopathy)
Clinical
♦
Complication of ocular diseases (chronic corneal inflammation, glaucoma) or systemic disorders
♦
Band of corneal opacification corresponding to the interpalpebral fissure
Microscopic
♦
Basophilic granules of calcium deposits in the Bowman membrane and superficial stroma (Fig. 25.19)
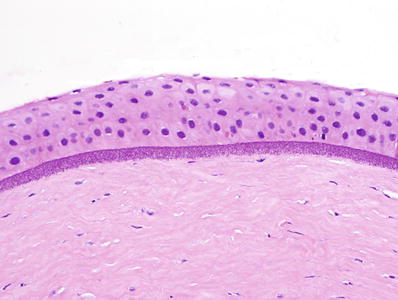

Fig. 25.19.
Band keratopathy: granular calcific deposits in the Bowman membrane and anterior stroma.
♦
Special stains for calcium may be obtained but are usually not necessary
Keloid of the Cornea
Clinical
♦
Marked thickening and opacification of the cornea
♦
Develops as a consequence of surgical or nonsurgical trauma
Microscopic
♦
Markedly hypertrophic scar of the corneal stroma
♦
Anterior corneal epithelium may become keratinized (exposure) (Fig. 25.20)
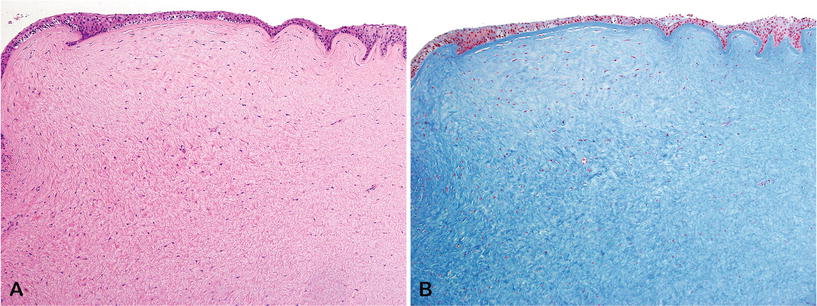

Fig. 25.20.
Keloid of the cornea following penetrating trauma, (A) H&E and (B) Masson trichrome.
♦
Other lesions may be observed histopathologically (i.e., iris synechiae, acute or chronic inflammation, previous wounds, etc.)
Salzmann Nodular Degeneration
Clinical
♦
Degenerative process of uncertain etiology
♦
Idiopathic or secondary to chronic corneal inflammation
♦
Usually unilateral but may be bilateral
♦
More common in women
♦
May affect the central or paracentral cornea
Microscopic
♦
Subepithelial nodules of dense collagen deposition
♦
Distort or replace the Bowman membrane and superficial stroma
♦
Overlying epithelium and its basement membrane may be thickened (Fig. 25.21)
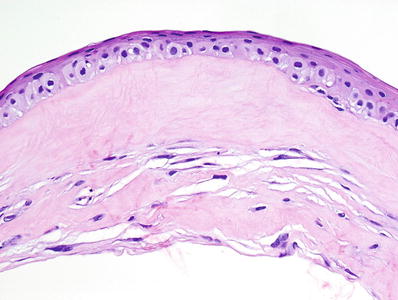

Fig. 25.21.
Salzmann nodular degeneration. Note the absence of the Bowman membrane and the compact collagenous deposition in the subepithelial stroma.
Spheroidal Degeneration (Labrador Keratopathy, Climatic Droplet Keratopathy, Actinic Keratopathy)
Clinical
♦
Usually develops as a consequence of prolonged sun exposure
♦
In some cases, it is a complication of chronic corneal inflammation
♦
Like calcific band keratopathy, it usually affects the area corresponding to the interpalpebral fissure
♦
Translucent yellow or golden-brown deposits in superficial cornea
Microscopic
♦
Spheroidal (drop-like) deposits of hyaline material
♦
Pale basophilic or amphophilic in H&E-stained sections (Fig. 25.22)
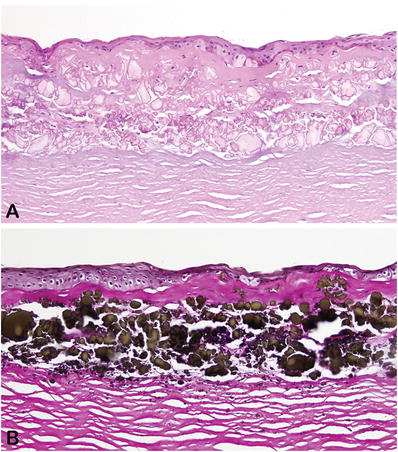

Fig. 25.22.
Severe spheroidal keratopathy associated with a dense fibrous pannus. (A) H&E and (B) Verhoeff-van Gieson.
♦
Strongly positive in sections stained for elastic fibers (Verhoeff–van Gieson stain)
♦
Often associated with variable degrees of scarring in the corneal stroma
♦
May coexist with calcific band keratopathy
Fuchs Dystrophy (Fuchs Endothelial Dystrophy, Cornea Guttata)
Clinical
♦
The most common corneal dystrophy
♦
Etiology: primary defect of the corneal endothelium
♦
Usually develops in late adult life
♦
Patients present with chronic corneal edema and bullous keratopathy
♦
Defects on posterior surface of the Descemet membrane are easily observed on slit-lamp examination
Microscopic
♦
Thickened Descemet membrane, best demonstrated in PAS-stained sections
♦
The Descemet membrane may have a laminated appearance
♦
Numerous wart-like or mushroom-like protrusions on posterior surface of the Descemet membrane (“guttae”) due to abnormal deposition of basement membrane material by the dysfunctional corneal endothelium (Fig. 25.23)
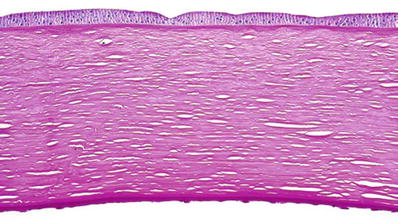

Fig. 25.23.
Fuchs dystrophy. The thickened Descemet membrane is studded with wart-like protrusions (“guttae”) on its posterior surface. PAS stain.
♦
The number of endothelial cells is decreased
♦
Melanin pigment granules from the iris may be observed in the cytoplasm of endothelial cells
♦
Changes due to chronic corneal edema and bullous keratopathy are usually observed
Granular Dystrophy
Clinical
♦
One of the classical dystrophies of the corneal stroma
♦
It is a hereditary condition transmitted as an autosomal dominant trait
♦
Usually develops in older adults
♦
Presents as multiple superficial opacities in the stroma of the cornea
♦
Visual acuity is minimally impaired
♦
Caused by a mutation in the TGFB1 gene (chromosome 5q31)
♦
The lesion recurs after corneal transplantation
Microscopic
♦
Multiple irregular deposits of a pale eosinophilic material in stroma
♦
Full thickness of the cornea may be affected
♦
Deposits stain bright red with the Masson trichrome and are negative with Congo red
♦
Ultrastructurally, the deposits appear as dense crystalloids, occasionally with vague periodicity
Macular Dystrophy



