Chapter 16 Extracellular Messengers
This chapter describes the metabolism of extracellular messengers. The actions of these agents on their target cells are discussed in Chapter 17.
Steroid hormones are made from cholesterol
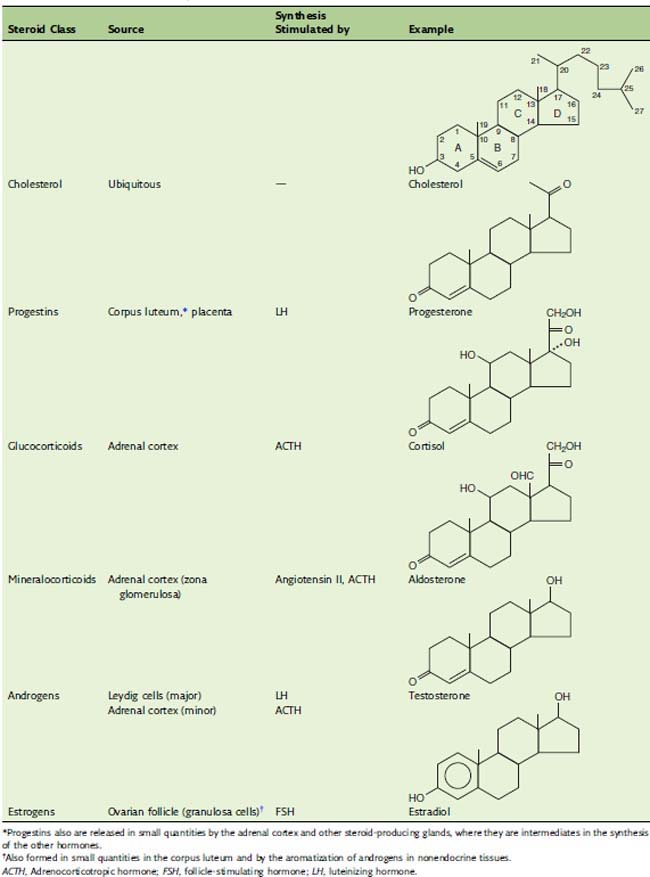
The steroids are classic hormones, synthesized in endocrine glands, and transported by the blood. The adrenal steroids regulate energy metabolism (glucocorticoids) and mineral balance (mineralocorticoids), and the gonadal steroids are concerned with sex. Table 16.1 lists representatives of the major classes of steroid hormones.
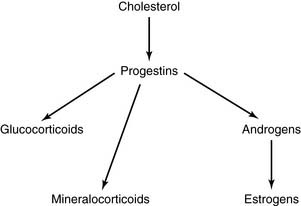
Progestins are the biosynthetic precursors of all other steroid hormones
The precursor relationships of the steroid hormones can be summarized as follows:
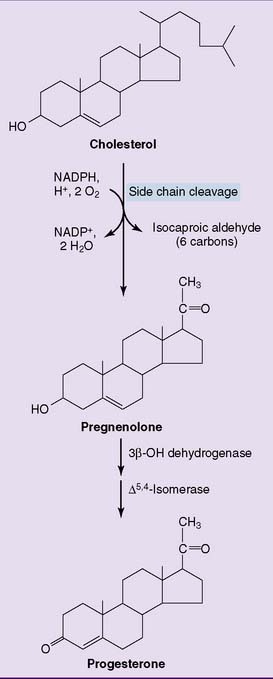
The very first reaction (Fig. 16.1) is catalyzed by the mitochondrial side chain cleavage enzyme, also known as desmolase. It hydroxylates carbons 20 and 22, followed by cleavage of the carbon-carbon bond. This reaction produces pregnenolone, which is converted to progesterone by a nicotinamide adenine dinucleotide (NAD)-dependent enzyme. Progesterone is the major product of the corpus luteum and the placenta. The other endocrine glands convert pregnenolone and progesterone to other steroid hormones.
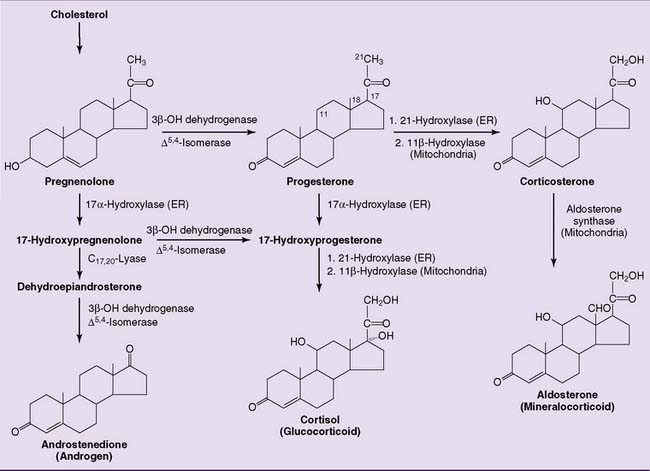
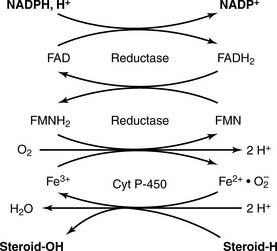
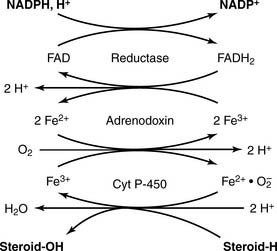
The adrenal cortex processes the progestins into corticosteroids, including the major glucocorticoid cortisol (10–20 mg/day) and the major mineralocorticoid aldosterone (0.10–0.15 mg/day). The most important reactions of corticosteroid synthesis (Fig. 16.2) are hydroxylations with the overall balance
CLINICAL EXAMPLE 16.1: Licorice-Induced Hypertension
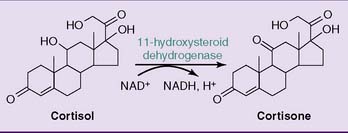
The plasma concentration is more than 100 times higher for cortisol than for aldosterone. At this high concentration, cortisol can activate mineralocorticoid receptors in the kidneys and cause the typical mineralocorticoid effects of sodium retention and potassium excretion. This is normally prevented by the NAD+-dependent enzyme 11β-hydroxysteroid dehydrogenase in the kidneys, which converts cortisol to cortisone, which no longer has mineralocorticoid activity (Fig. 16.3).
The adrenal cortex contributes approximately 20 mg of androgen per day. Adrenal androgens include dihydroepiandrosterone and androstenedione, but only trace amounts of testosterone. The adrenal androgens have a keto group instead of a hydroxy group at C-17 and therefore are far less potent than testosterone, but they are a major source of androgenic activity in females (Figs. 16.2 and 16.4).
Abnormalities in androgen synthesis lead to disorders of sexual development (Clinical Examples 16.2 and 16.3).
CLINICAL EXAMPLE 16.2: Congenital Adrenal Hyperplasia
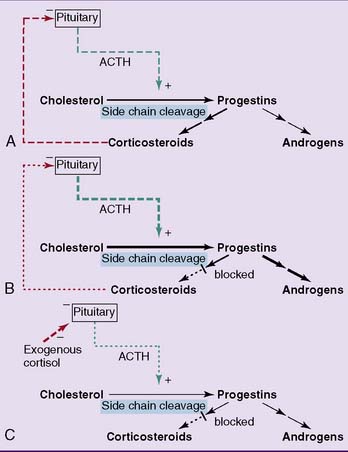
In the adrenal cortex, 21-hydroxylase and 11β-hydroxylase are required for the synthesis of corticosteroids but not androgens (see Fig. 16.2). Recessively inherited deficiencies of either of these enzymes leads to congenital adrenal hyperplasia, also known as adrenogenital syndrome (incidence 1:10,000, 90% of this is 21-hydroxylase deficiency).
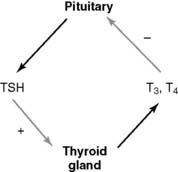
Ordinarily, the desmolase reaction in the adrenal cortex is stimulated by adrenocorticotropic hormone (ACTH) from the pituitary gland, and the glucocorticoids inhibit ACTH release. In adrenogenital syndrome, the deficiency of glucocorticoids enhances ACTH release. The excess ACTH causes adrenal hyperplasia and stimulates the desmolase reaction. With the pathway of corticosteroid synthesis blocked, the overproduced progestins are diverted into androgen synthesis (Fig. 16.5). This disorder produces ambiguous external genitalia in girls and precocious puberty in boys.
Thyroid hormones are synthesized from protein-bound tyrosine
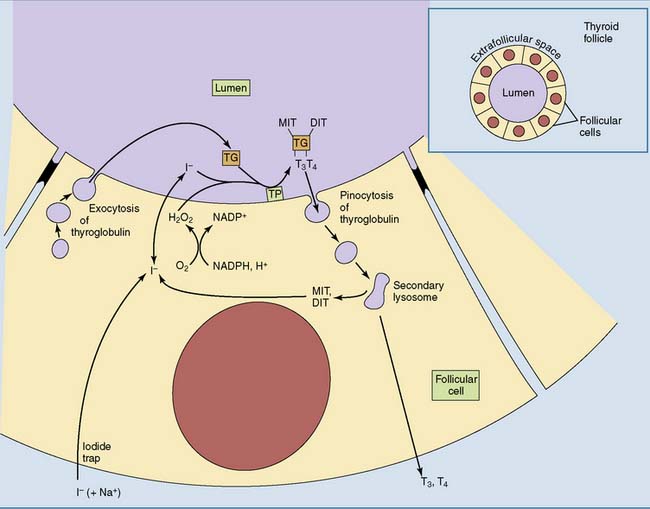
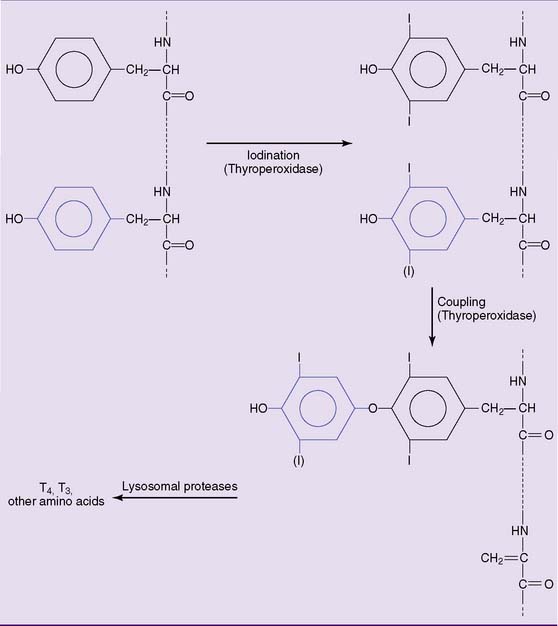
Iodide enters the lumen of the thyroid follicle by facilitated diffusion from the follicular cells (Fig. 16.6). Here it meets the second ingredient for hormone synthesis, thyroglobulin. This large glycoprotein (two subunits, 2 × 330,000 D) is secreted into the lumen of the thyroid follicle by the follicular cells. Up to 40 tyrosine side chains in thyroglobulin become iodinated, but only 8 to 10 of them are processed to the active hormones.
Iodination of the tyrosine side chains requires the oxidation of iodide by the heme-containing enzyme thyroperoxidase on the apical (luminal) surface of the plasma membrane (see Fig. 16.6). The iodine reacts with tyrosine side chains, and the coupling of two iodinated tyrosines produces the protein-bound hormones (Fig. 16.7).
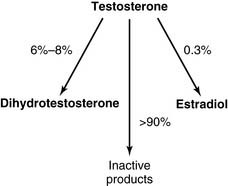
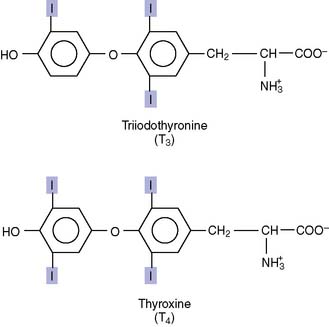
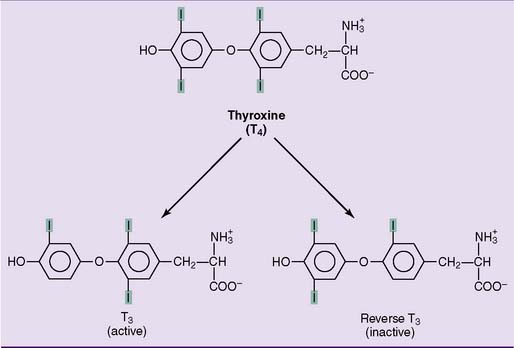
Ninety percent of the released hormone is T4, and 10% is T3. In the target tissues, some T4 is converted to active T3, and some is converted to the inactive reverse T3 (Fig. 16.8). Eighty percent of the circulating T3 does not come directly from the thyroid gland but is produced from T4 in peripheral tissues. Of the two hormones, T3 is about four times more potent than T4. Therefore T4 is a prohormone for the more potent T3, much as testosterone is a prohormone for the more potent dihydrotestosterone.
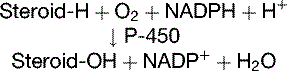
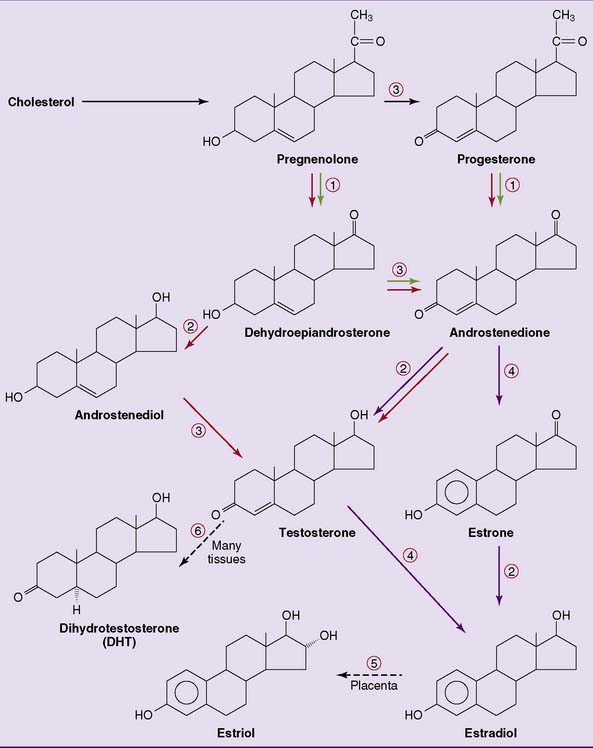
 , All steroid-producing cells;
, All steroid-producing cells;  , ovary, theca cells;
, ovary, theca cells;  , ovary, granulosa cells;
, ovary, granulosa cells;  , testis;
, testis;  , 17α-hydroxylase/C17,20-lyase;
, 17α-hydroxylase/C17,20-lyase;  , 17β-hydroxysteroid dehydrogenase;
, 17β-hydroxysteroid dehydrogenase;  , 3β-dehydrogenase and Δ5,4-isomerase;
, 3β-dehydrogenase and Δ5,4-isomerase; 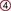 , aromatase;
, aromatase;  , 16α-hydroxylase;
, 16α-hydroxylase;  , 5α-reductase.
, 5α-reductase.