Fig. 3.1
Schematic image of the increased surface area generated by the micro-roughening procedure
Along with implant macro-geometry, the increased kinetic friction naturally provides higher implant primary stability [7], which is equivalent to the lower micro-motion of the implant in the bone. The high primary stability of the implant provides a stable host bed, which allows growth factors and cells to successfully adhere to the implant surface.
From a biological viewpoint, the moderately roughened surface micro-topography seems to modulate the cellular events towards a more osteogenic atmosphere [8]. It has been reported that the surface micro-roughness is a regulatory factor for the production of growth factors, cell attachment, proliferation, differentiation, and mineralization [9, 10]. Interestingly, the scientific evidence from in vitro studies suggests that osteogenic cells possess a topography cognitive mechanism to micron or to sub-micron structures [11–13]. Ismail et al. have shown that the viability and attachment of osteoblasts to commercially available implant surfaces had altered depending on the micro-topography (in this case, different size microgrooves) [11]. What is more interesting is that these cells seem to follow the microgrooves created, which provided further stability of the cell attachment and improved osteogenesis [14]. The so-called contact guidance is a typical example of a micro-topography influencing the biological outcomes of osteogenic cells, which also strongly indicates the importance of micro-topography on biomaterial surfaces.
Thus, this chapter focuses on the effect of micro-topography on osseointegration from both experimental and clinical aspects. Furthermore, the different surface modification methodologies will be introduced to deepen the knowledge of implant surface topography, which should help the readers to better understand the following chapters on this topic.
Turned Implant Surfaces
The osseointegration implants initially utilized by the Swedish and the Swiss groups were the turned (or the so-called machined) implants, which in reality has the longest clinical documentation. The term ‘machined’ should be avoided as much as possible in this book since it is well known that implants are manufactured by a turning process in a turning machine. The term ‘machined’ could be any surface finish, since as long as a ‘machine’ is involved in the manufacturing process, the surface could be called ‘machined’; thus, it is clear that the terminology is not suitable especially for a book focusing on implant surfaces. For example, implants that have a mechanically polished surface finish are often rightfully called ‘machined’ and compared with turned ‘machined’ surfaces. However, these polished implants possess a much smoother surface topography in the micro-level compared to the traditional Brånemark turned implant surfaces; thus, the comparison is not correct from a topographical point of view. Turned surface implants have indeed a long clinical history. Until the mid-1990s the turned implants dominated the market. One of the first long-term documentation concerning the survival of the turned osseointegrated implants stated that 81 % of the maxillary implants and 91 % of the mandibular fixtures remained stable after a 15-year period [15]. Attard and Zarb had replicated the studies performed in Sweden and reported in their prospective study that over a 20-year period (range 18–23 years), the implant success rate with fixed prosthesis in edentulous patients was 87 % [16]. These studies clearly highlight that the turned implants present good prognosis over a long period. According to Wennerberg and Albrektsson [4], these once commercially available turned Brånemark implants had a surface topographical value of Sa of 0.9 μm and an Sdr of 34 % [4]. Thus, it can be said that the effect of surface micro-topography may have contributed in the long-term clinical success of the turned commercially available implants. However naturally, their definitive role during functional loading over a long period is difficult to distinguish since implant success is a complex blend of multiple factors.
Rough Implant Surfaces
In general, the traditional Brånemark turned implants placed in the mandible, or the maxilla, followed a 2-stage protocol, which allowed the implants to heal (osseointegrate) [17]. Clinically, it has been suggested that the turned implants required a healing period of 3 months in the mandible and 6 months in the maxilla. This was probably one of the reasons why the so-called rough implant surfaces appeared on the market in order to provide higher grip between the implant surface and the bone. It can be said that back in the days when the development of these surfaces took place, there was little evidence on what is the optimal roughness. Although in general, from a mechanical viewpoint, the rougher surfaces have been known to provide higher stability, the clinical performance of these surfaces was without proper evidence. More on the optimal roughness, namely, the moderately roughened surface, will be introduced later in this chapter.
In this section, the two major surface-roughening procedures, i.e. evidence with regard to the titanium plasma spraying technique and hydroxyapatite coating technique, will be briefly introduced.
Titanium Plasma-Sprayed Rough Implant Surfaces
The plasma spray technique, which yields a bumpy surface configuration [3], was introduced to roughen the titanium outermost layer so that the implants allegedly would osseointegrate faster than the turned implants [18]. Wennerberg and Albrektsson [3] have summarized different implant surface topographies in their review and have stated that the surface roughness of the plasma-sprayed implants possess a surface roughness of approximately Ra 4–5 μm [3]. Although the Ra as we know is a two-dimensional parameter and cannot be directly correlated to the three-dimensional Sa, it is evident that the surface topography of the plasma-sprayed implant surfaces possesses quite a rough topography compared to the turned implant surfaces.
The rougher surface generated by the titanium plasma spray (TPS) technique seemed to accelerate osseointegration in some animal studies [18–20]; on the contrary, some studies including a clinical 5-year randomized, control clinical study by Roccuzzo et al. indicated no major benefits of the TPS surfaces [21–23]. Moreover, numerous studies indicated that there were more complications with these rough implant surfaces compared to the less rough or turned surfaces and caused more marginal bone loss [19, 24–28]. Especially with periodontally susceptible patients, De Boever et al. indicated that the survival of the TPS implants significantly decreased compared to the less rough implant surfaces [29]. This trend further worsens with periodontally susceptible patients with a smoking habit. Aglietta et al. have reported that patients in this group after 10 years showed an average marginal bone loss of 2.5 mm [30]. Although the cumulative survival rate (CSR) was 100 %, one can question the success of the implant, and this can be regarded as one of the drawbacks of presenting survival rates.
Rough and Thick Hydroxyapatite Coatings
Another type of implant surface that will be introduced in this section is the thick hydroxyapatite-coated implant with a rough microsurface, most of which are no longer commercially available. These surfaces have been well described by Wennerberg et al. [31], in their study observing the design and topographical characteristics of 13 different implant systems [31]. The authors found that the hydroxyapatite implant surface presented the highest surface roughness compared to the other textured surfaces.
Experimentally, this surface has proven to be bioactive and promotes osteogenesis especially in the short term [32–36]. On the contrary, Gottlander et al. have suggested that at relatively longer healing periods in the animal study, the outermost layer of the hydroxyapatite and the bone in proximity to the surface were affected by a macrophage-induced resorption [37]. Moreover, Registad et al. have reported a time course coat flaking and delamination of the hydroxyapatite with multinucleated giant cell activity and bone resorption [38]. This phenomenon is further evident with the clinical performance of these implants as it seems that the clinical success in the long term was less favourable with many of them resulting in marginal bone loss [39].
Albrektsson et al. have reported the existence of loose hydroxyapatite particles in the tissue around clinically failed hydroxyapatite implants [40]. As indicated by the same author, the surface roughness of these implants are normally Sa = 2.0 μm or higher, and the coat thickness is between 80 and 100 μm; thus, the initial stability and fit may seemingly be excellent [41]. However, it is also suggested that these features may actually act against their clinical prognosis. Insertion protocols and functional loading may promote loosening or breakage of the particles, which induces the foreign body reaction. Furthermore, hydroxyapatite rough surfaces may be a host bed for numerous microorganisms, which may be one of the reasons for implant complications.
Moderately Roughened Implant Surfaces
As of 2014, a majority of the implant surfaces have textured micro-topographies. In principle, the turned implants as a substrate are treated with different roughening procedures such as sand blasting [42–44], acid etching [45–47], anodic oxidation [48–50], and laser etching [51–53]. More importantly, a majority of the commercially available implants of today are strategically roughened in the micro-level to present the optimal bone responses. The moderately roughened micro-roughness, as mentioned in the introduction, is the major key to the success of the implant from a surface topography viewpoint.
This success stands on the knowledge that bone responds in a different manner to different surface topographies. Wennerberg et al. have shown that implant surfaces blasted with titania particles of 25 μm and alumina particles of 75 μm presented higher bone-to-implant contact than the turned implants. For the roughness parameters, especially the average height deviation, the Ra presented differences between the turned and the blasted surfaces, with the blasted surface presenting two to three times higher topographical values (Ra = 0.4 μm, and Ra = 0.9–1.3 μm, respectively) [54]. It was suggested in another study from Wennerberg et al. that the mode of roughening, in other words, the material used to roughen, did not play a significant role on the biological outcome and the alterations in surface topography were the influential factors with regard to osseointegration (direct bone-to-implant contact) [55, 56]. Although these experimental results suggested a linear relation between the surface roughness and osseointegration, another report from Wennerberg et al. has suggested that highly increased surface roughness presents lower bone-to-implant interactions. In brief, the implants blasted with 250 μm particles (average surface height deviation Sa = 1.88 μm) presented significantly lower bone-to-implant contact than the implants blasted with 25 μm particles (average surface height deviation Sa = 1.16 μm). Interestingly, the removal torque values and the bone area presented no significant differences, which combined with the bone-to-implant contact suggests a non-linear relation between surface roughness and osseointegration [57]. As shown in Fig. 3.2, which is a summary of the series of articles presented with regard to this topic by Wennerberg et al., there seems to be a range of surface roughness that presents the strongest bone responses, and as stated in the introduction, many of the commercially available implants of today possess a moderately roughened micro-topography.
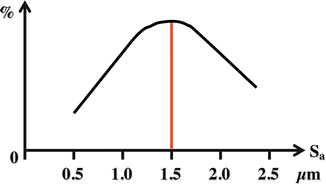

Fig. 3.2
Summary of the thesis work presented by Ann Wennerberg suggesting that the degree of osseointegration can vary depending on the surface micro-topography. It has been suggested that the moderately roughened implant micro-topography presents the strongest bone responses
Clinically, we know from experience that the moderately roughened implant surfaces osseointegrate faster and the time to functionally load has significantly reduced compared to the turned implant surfaces. Although it is difficult to prove that the implants are osseointegrating faster in the patients’ bone, the alterations in loading protocols (from delayed to early or immediate) and their success clearly suggest the effects and benefits of the moderately roughened implants [58].
With regard to the clinical outcomes of the implants possessing moderately roughened surface topography after a 5-year period, the prognosis has been reported to present good outcomes. Gotfredsen and Karlsson have reported that commercially available implants with a moderately roughened surface topography presented 100 % survival after 5 years with low levels of marginal bone loss with a fixed partial prostheses as superstructures [59]. Akogulu et al. reported that three implants from different manufactures all possessing moderately roughened surfaces presented no differences in survival rates after 5 years (100 %) with marginal bone loss less than 0.4 mm with an overdenture reconstruction in the mandible.
When compared to the turned implants, the long-term implant survival of the moderately roughened implants presented no significant differences, if the implants are placed in sites such as fully healed sites or sites with good bone quality [60–62]. Thus, it seems that the moderately roughened implant surfaces do not present significant differences compared to the turned surfaces in normal situations; however, in compromised situations such as in poor quality bone, or in irradiated bone, the moderately roughened implants present their benefits. Khang et al. conducted a multicentre study testing the success of turned and dual acid-etched surfaces and reported that the success rates were notably higher for the dual acid-etched surfaces compared to the turned surfaces in poor bone quality conditions [63]. Pinholt has reported that in grafted maxillary bone, the moderately roughened implant surfaces outperformed the turned implant surfaces in terms of implant survival [64]. Buddula et al. investigated the differences in implant survival after 5 years using turned or moderately roughened implant surfaces in sites where radiation of at least 50 Gy was irradiated [65]. The results presented significantly higher survival rates for the moderately roughened implants both in the mandible and maxilla. In addition, a recent paper summarized 10 different non-controlled studies of moderately rough implant and found those to present a combined failure and peri-implantitis frequency within 5 % if followed up for 10 years or longer [66].
Concluding Remarks
This chapter focused on the importance of implant micro-topography on osseointegration and the clinical success of the osseointegrated oral implants. It is quite evident that the treatment modalities have changed due to the advancements in the surface micro-topography of the implant and there is a tendency to shorten the total treatment period. However, it is also important to understand and respect the biological phenomena since the bone cannot be formed in short healing periods or the bone can easily be damaged by coarse surgical or prosthetic procedures. Moderately roughened implant surfaces have been proven to present the most optimal clinical outcomes. To date, there is no evidence that these rough surfaces act negatively against bacterial infection and reducing the surface roughness of the implants could cause negative biologic reactions; thus, this trend should be cautiously observed.
References
1.
2.
Albrektsson T, Wennerberg A. Oral implant surfaces: part 1–review focusing on topographic and chemical properties of different surfaces and in vivo responses to them. Int J Prosthodont. 2004;17:536–43.PubMed
3.
Wennerberg A, Albrektsson T. Effects of titanium surface topography on bone integration: a systematic review. Clin Oral Implants Res. 2009;20:172–84.PubMedCrossRef
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


