28
Estrogens, Progestins, Androgens, and Contraception
This chapter will be most useful after having a basic understanding of the material in Chapter 40, Estrogens and Progestins, Chapter 41, Androgens, and Chapter 66, Contraception and Pharmacotherapy of Obstetrical and Gynecological Disorders in Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 12th Edition. In addition to the material presented here, these chapters in the 12th Edition contain:
• The biosynthesis and physiological and pharmacological actions of the estrogens, progestins, and androgens
• A detailed discussion of gonadotropin and gonadal steroid concentrations during the menstrual cycle, the effects of cyclical gonadal steroids on the reproductive tract, and the metabolic effects of estrogens, progestins, and androgens
• A detailed discussion of fertility induction and the general principles of drug therapy of pregnant women, and the prevention or arrest of preterm labor
• A detailed discussion of the secretion and transport of testosterone, the physiological and pharmacologic effects of androgens, and the effects of androgens at different stages of life
LEARNING OBJECTIVES
 Describe the mechanisms of action of estrogens, progestins, and androgens.
Describe the mechanisms of action of estrogens, progestins, and androgens.
 Understand the therapeutic use and side effects of postmenopausal hormone replacement therapy.
Understand the therapeutic use and side effects of postmenopausal hormone replacement therapy.
 Understand the therapeutic uses and side effects of contraceptive therapy.
Understand the therapeutic uses and side effects of contraceptive therapy.
 Describe the therapeutic use of selective estrogen receptor modulators, antiestrogens, and aromatase inhibitors.
Describe the therapeutic use of selective estrogen receptor modulators, antiestrogens, and aromatase inhibitors.
 Describe the use of testosterone in the treatment of male hypogonadism.
Describe the use of testosterone in the treatment of male hypogonadism.
 Describe the use of androgen receptor antagonists and 5α-reductase inhibitors to treat metastatic prostate cancer and benign prostatic hypertrophy, respectively.
Describe the use of androgen receptor antagonists and 5α-reductase inhibitors to treat metastatic prostate cancer and benign prostatic hypertrophy, respectively.
 Understand the side effects of the 17α-alkylated androgens used by athletes to enhance performance.
Understand the side effects of the 17α-alkylated androgens used by athletes to enhance performance.
DRUGS INCLUDED IN THIS CHAPTER
Anabolic steroids (various 17α-alkylated androgens)
Anastrozole (ARIMIDEX)
Bicalutamide (CASODEX, others)
Clomiphene (CLOMID, SERPPHENE, others)
Conjugated equine estrogens (PREMARIN)
Conjugated estrogens + medroxyprogesterone acetate (PREMPRO, PREMPHASE)
Dutasteride (AVODART)
Estradiol + drospirenone (ANGELO)
Estradiol + norethindrone (PREFEST)
Estradiol cypionate (DEPO-ESTRADIOL, others)
Estradiol micronized (ESTRACE, others)
Estradiol nonmicronized (FEMTRACE)
Estradiol topical gel (ESTROGEL)
Estradiol transdermal (ALORA, CLIMERA, ESTRADERM, VIVELLE, others)
Estradiol vaginal ring (ESTRING)
Estradiol vaginal tablets (VAGIFEM)
Estradiol valerate (DELESTROGEN, others)
Estrogen esterified esters (MENEST)
Estropipate (ORTHO-EST, OGEN, others)
Ethinyl estradiol + norethindrone (FEMHRT)
Exemestane (AROMASIN)
Finasteride (PROSCAR)
Flutamide (EULEXIN)
Formestane
Fulvestrant (FASLODEX)
Letrozole (FEMARA)
Medroxyprogesterone acetate (MPA; PROVERA, DEPO-PROVERA)
Megestrol acetate (MEGACE, others)
Mifepristone (RU 38486; MIFEPREX)
Nilutamide (NILADRON)
Progesterone micronized (PROMETRIUM)
Progesterone slow-release intrauterine device (PROGESTASERT)
Progesterone vaginal gel (CRINONE, PROCHIEVE)
Progesterone vaginal insert (ENDOMETRIN)
Raloxifene (EVISTA)
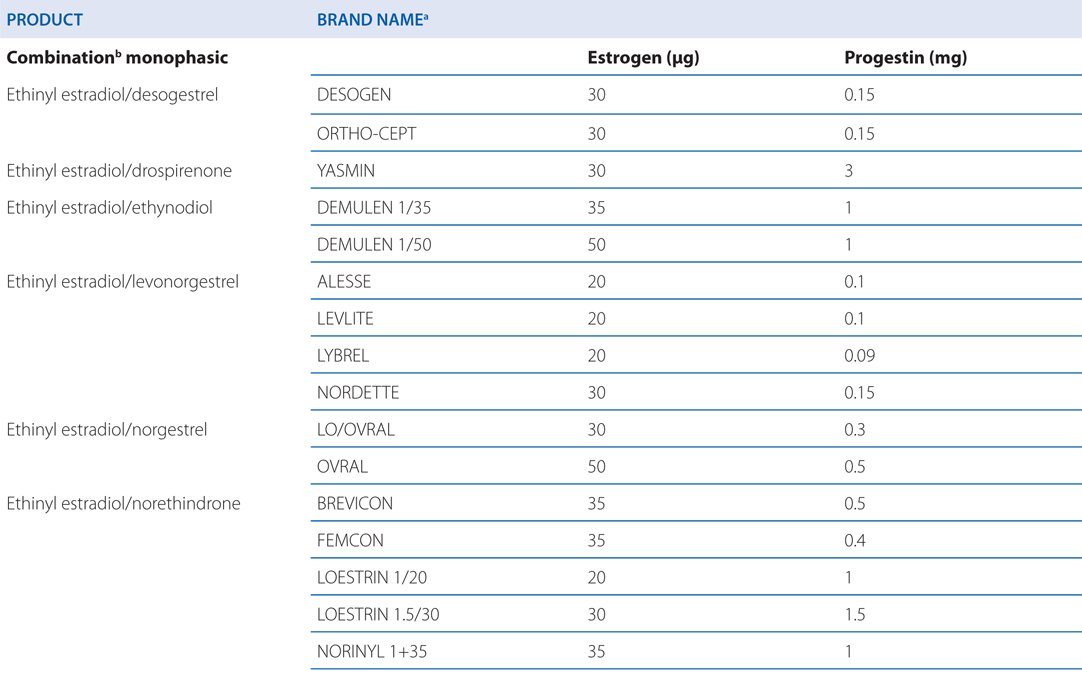
See Table 28-1 for brand names and formulations of oral contraceptives
TABLE 28-1 Brand Names and Formulations of Oral Contraceptives
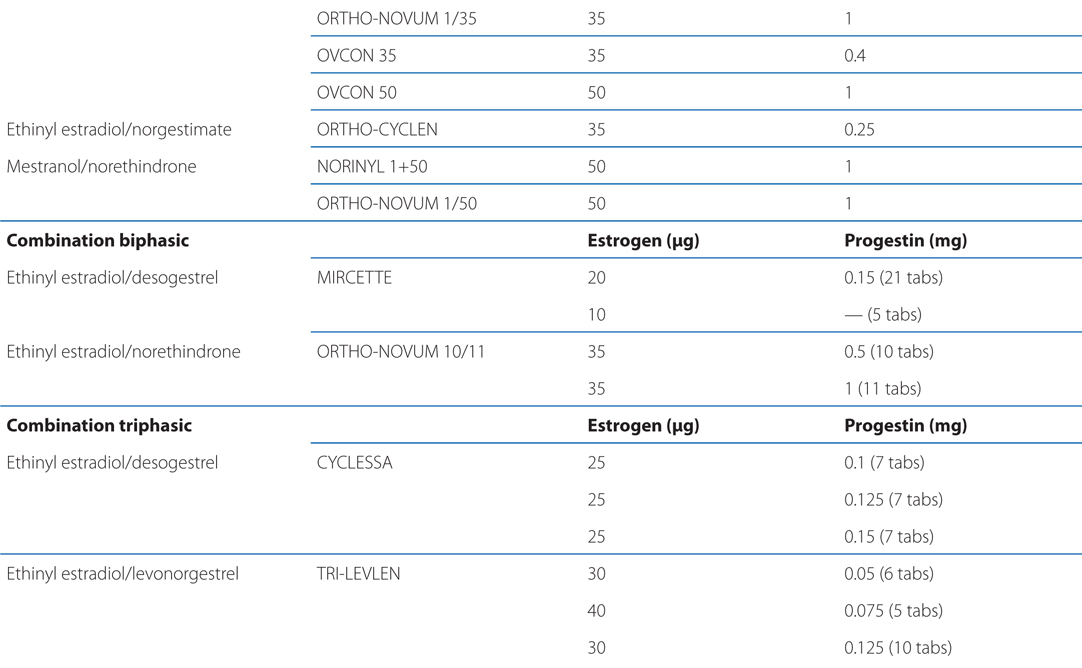
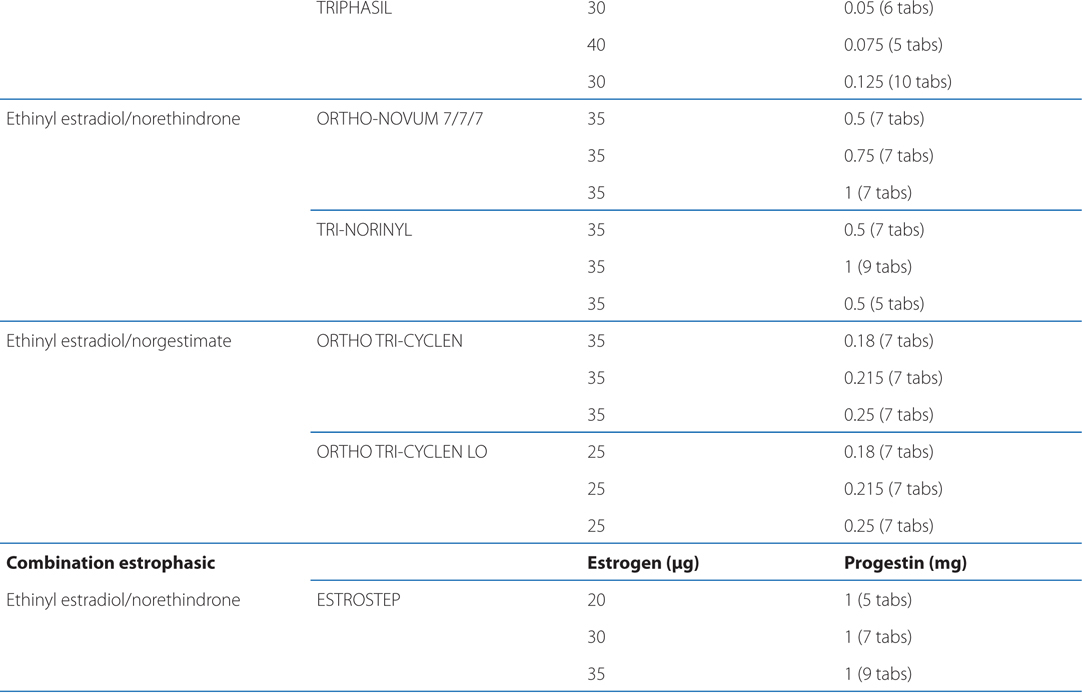
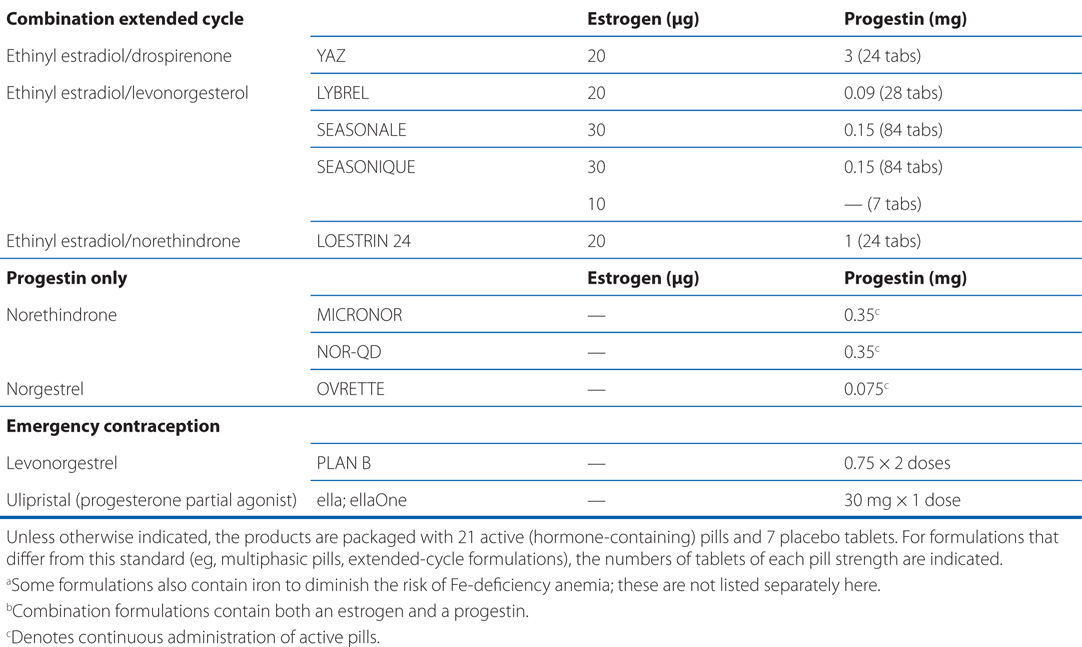
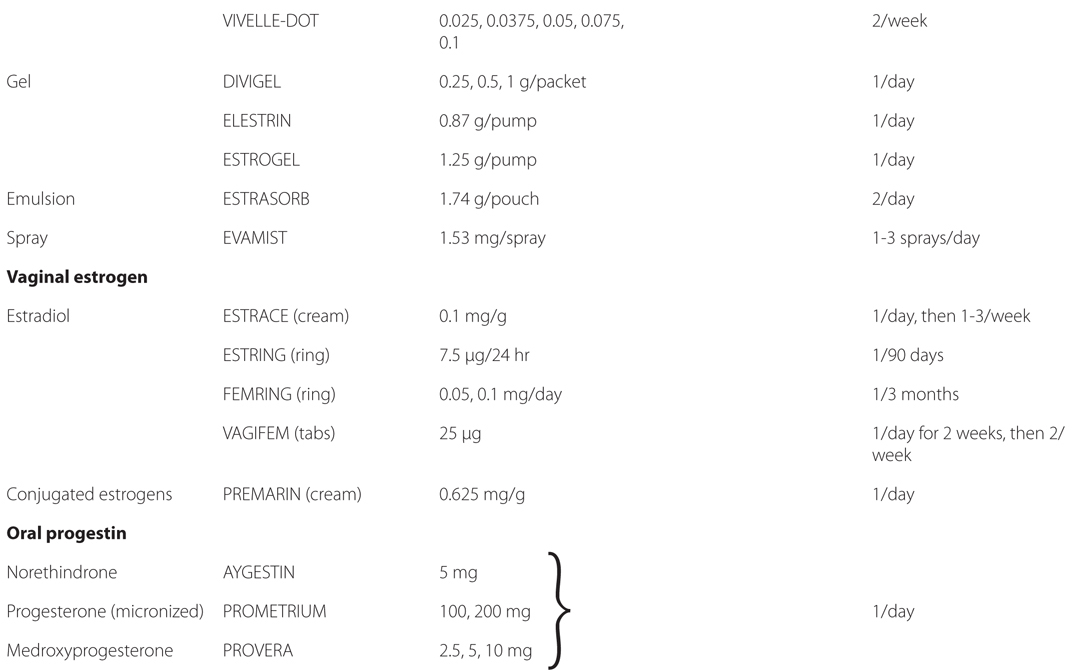
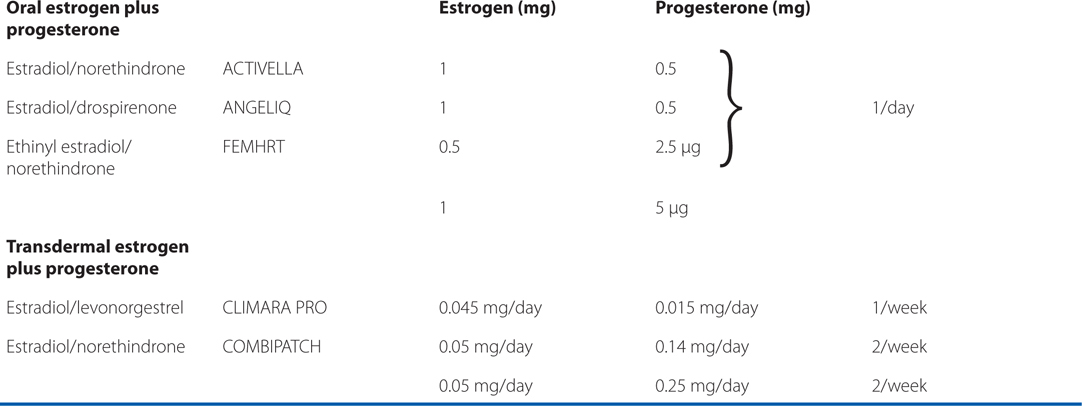
See Table 28-2 for brand names and formulations of agents used for hormone replacement therapy
TABLE 28-2 Brand Names and Formulations of Agents Used for Hormone Replacement Therapy
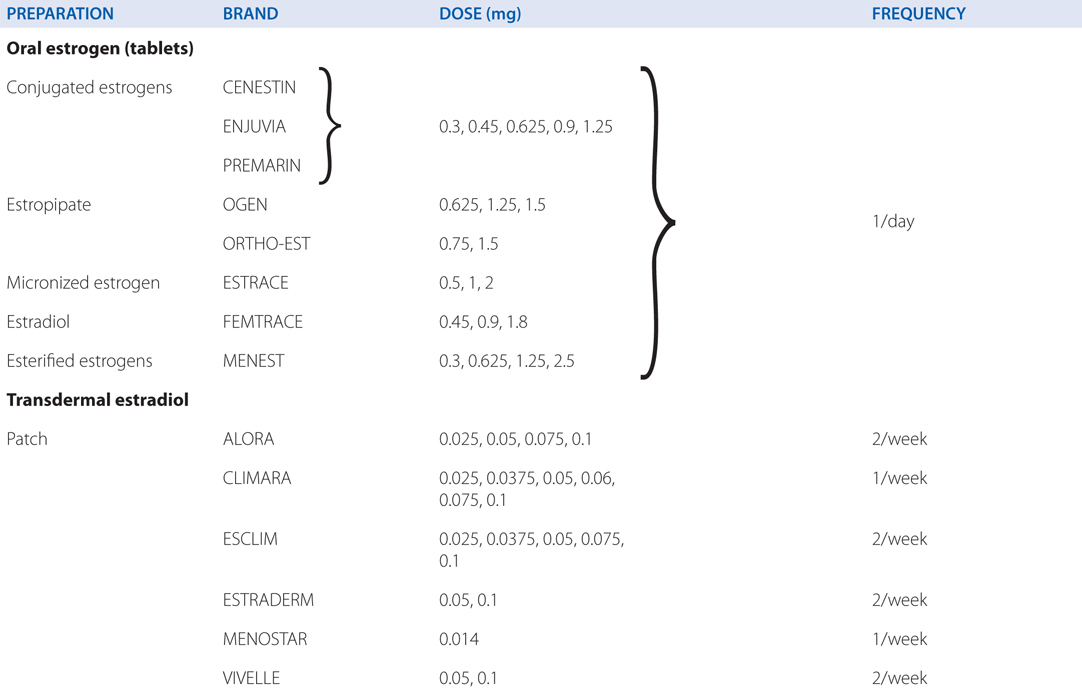
Synthetic conjugated estrogens (CENESTIN; ENJUVIA)
Tamoxifen
Testosterone (HISTERONE, others)
Testosterone buccal tablet (STRIANT)
Testosterone cypionate (DEPO-TESTOSTERONE, others)
Testosterone enanthate (DELATESTRYL, others)
Testosterone gel (ANDROGEL, TESTIM)
Testosterone patch (ANDRODERM)
Testosterone undecanoate (ANDRIOL)
Toremifene (FARESTON)
Ulipristal (ELLA, ELLAONE)
MECHANISMS OF ACTION OF ESTROGENS, PROGESTINS, AND ANDROGENS
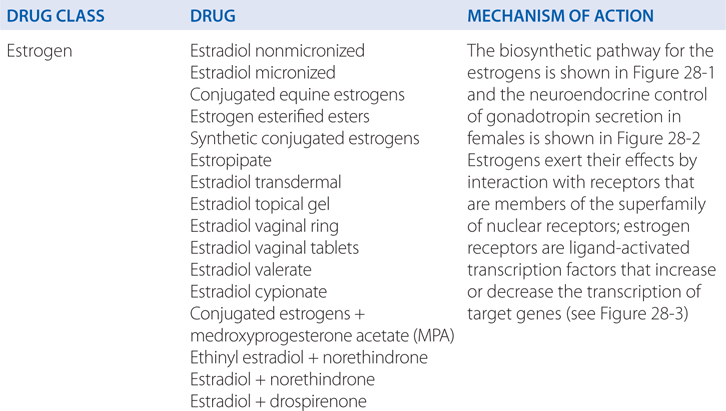
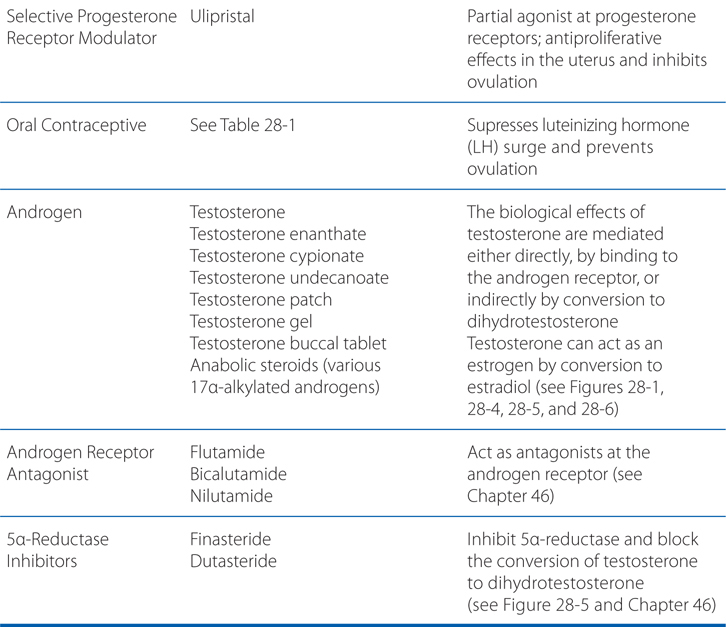
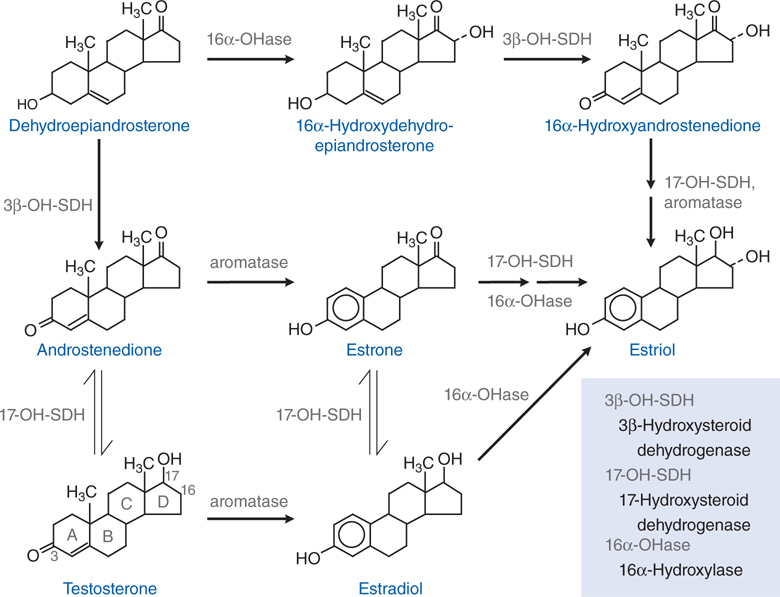
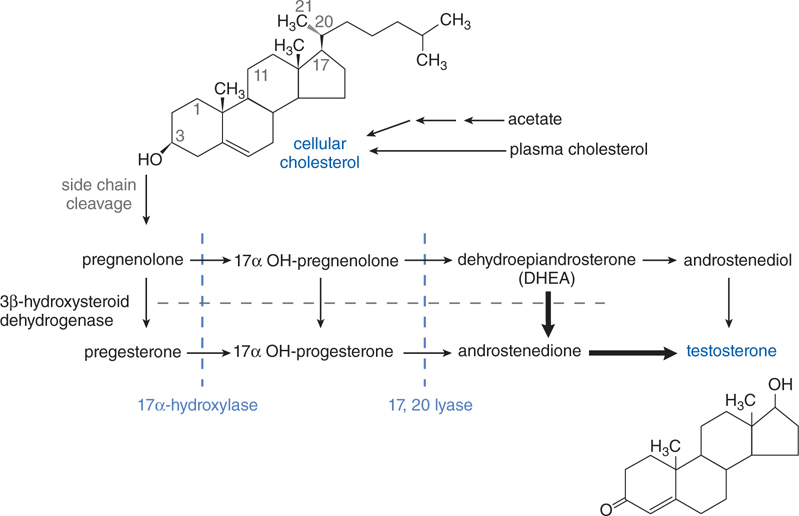
FIGURE 28-1 The biosynthetic pathway for the estrogens.
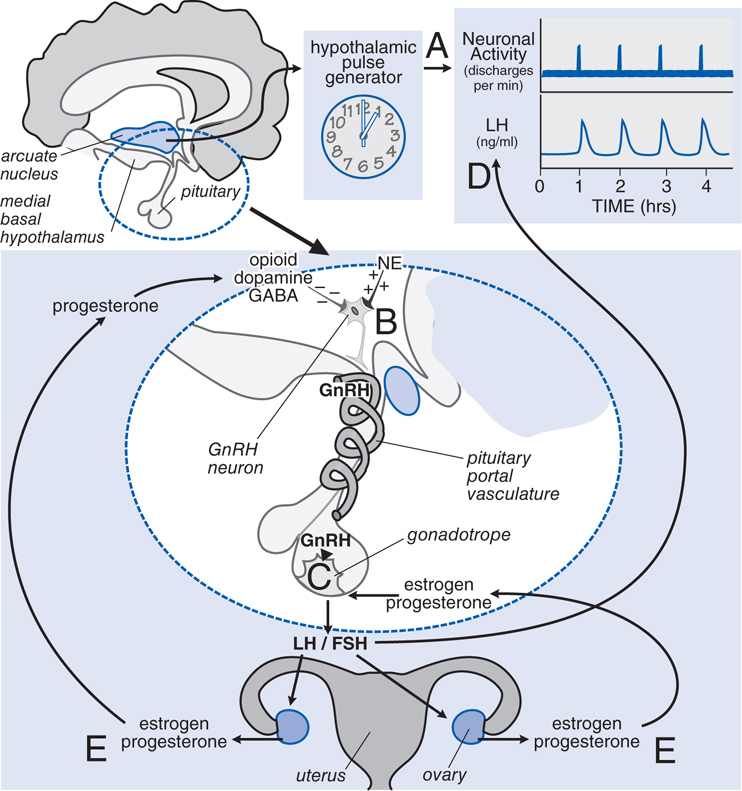
FIGURE 28-2 Neuroendocrine control of gonadotropin secretion in females. The hypothalamic pulse generator located in the arcuate nucleus of the hypothalamus functions as a neuronal “clock” that fires at regular hourly intervals. A. This results in the periodic release of gonadotropin-releasing hormone (GnRH) from GnRH-containing neurons into the hypothalamic-pituitary portal vasculature B. GnRH neurons receive inhibitory input from opioid, dopamine, and GABA neurons and stimulatory input from noradrenergic neurons (NE, norepinephrine). The pulses of GnRH trigger the intermittent release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) from pituitary gonadotropes C, resulting in the pulsatile plasma profile D. FSH and LH regulate ovarian production of estrogen and progesterone, which exert feedback controls E.
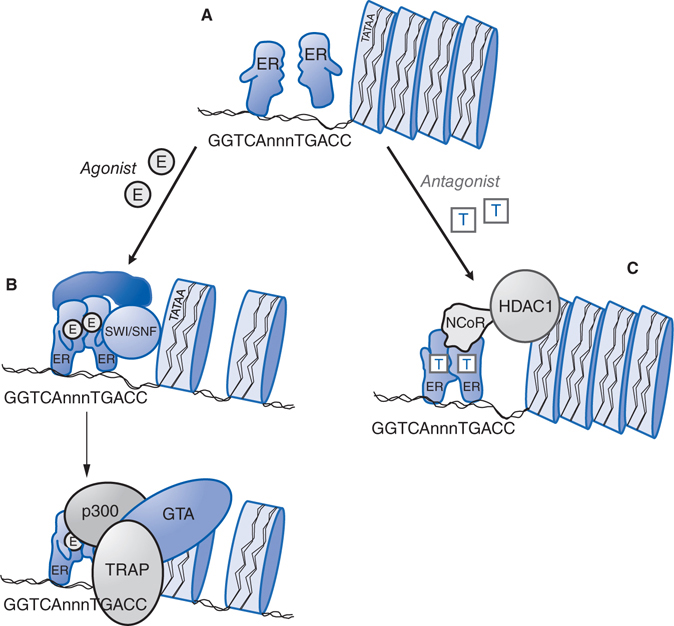
FIGURE 28-3 Molecular mechanism of action of nuclear estrogen receptor. A. Unliganded estrogen receptor (ER) exists as a monomer within the nucleus. B. Agonists such as 17β-estradiol bind to the ER and cause a ligand-directed change in conformation that facilitates dimerization and interaction with specific estrogen response element (ERE) sequences in DNA. The ER-DNA complex recruits coactivators such as SWI/SNF that modify chromatin structure, and coactivators such as steroid-receptor coactivator-1 (SRC-1) that has histone acetyltransferase (HAT) activity and that further alters chromatin structure. This remodeling facilitates the exchange of the recruited proteins such that other coactivators (eg, p300 and the TRAP complex) associate on the target gene promoter and proteins that comprise the general transcription apparatus (GTA) are recruited, with subsequent synthesis of mRNA. C. Antagonists such as tamoxifen (T) also bind to the ER but produce a different receptor conformation. The antagonist-induced conformation also facilitates dimerization and interaction with DNA, but a different set of proteins called corepressors, such as nuclear-hormone receptor corepressor (NcoR), are recruited to the complex. NcoR further recruits proteins such as histone deacetylase I (HDAC1) that act on histone proteins to stabilize nucleosome structure and prevent interaction with the GTA.
FIGURE 28-4 Pathway of synthesis of testosterone in the Leydig cells of the testes. In Leydig cells, the 11 and 21 hydroxylases (present in adrenal cortex) are absent but CYP17 (17 α-hydroxylase) is present. Thus androgens and estrogens are synthesized; corticosterone and cortisol are not formed. Bold arrows indicate favored pathways.
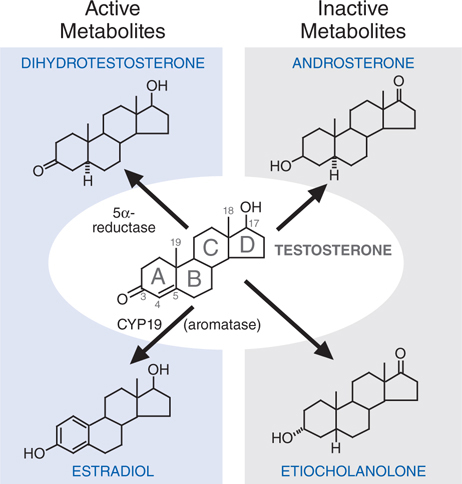
FIGURE 28-5 Metabolism of testosterone to its major active and inactive metabolites.
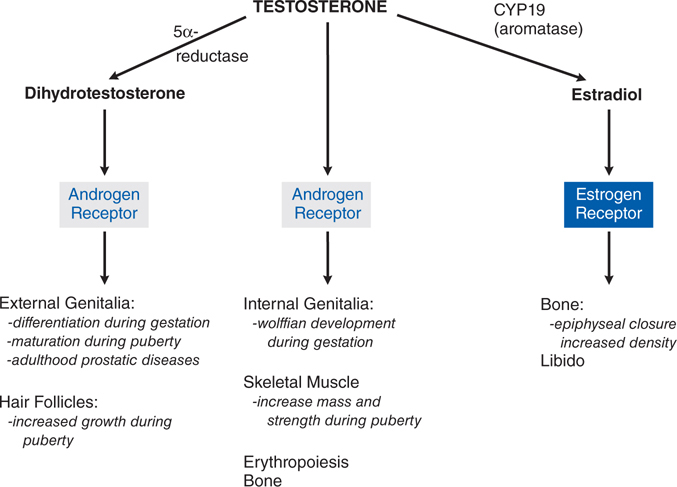
FIGURE 28-6 Direct effects of testosterone and effects mediated indirectly via dihydrotestosterone or estradiol.
A 49-year-old woman has been 6 months without menstruation. She is now experiencing severe “hot flashes” that are impacting her daily life. She would like some therapy that would make her more comfortable.
a. What hormone replacement therapy is available for her?
Table 28-2 lists the brand names and formulations used for hormone replacement therapy. Regardless of the specific agent or regimen, menopausal hormone therapy with estrogens should use the lowest dose and shortest duration necessary to achieve an appropriate therapeutic goal.
b. She is being treated with a combination estrogen/progesterone product (ethinyl estradiol/norethindrone, FEMHRT). Why is the progesterone added to the estrogen?
The use of unopposed estrogen for hormone treatment in postmenopausal women increases the risk of endometrial carcinoma by 5- to 15-fold. This increased risk can be prevented if a progestin is coadministered with the estrogen.
c. What are the mechanisms of action estrogen and progesterone?
Figure 28-3 describes the mechanism of action of a nuclear estrogen receptor. Estrogen and progesterone receptors are ligand-activated transcription factors that increase or decrease the transcription of target genes.
d. What are the risks of hormone replacement therapy that should be discussed with the patient?
Oral estrogens (and estrogen-progestin combinations) have a small but significant risk of thromboembolic disease in healthy women and in women with preexisting cardiovascular disease.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree