THE ESOPHAGUS
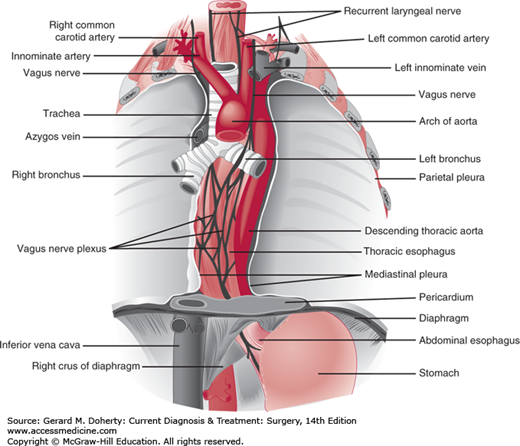
The esophagus (Figure 20–1) is a muscular tube that serves as a conduit for the passage of food and fluids from the pharynx to the stomach. It originates at the level of the sixth cervical vertebra, posterior to the cricoid cartilage. In the thorax, the esophagus passes behind the aortic arch and the left main stem bronchus, enters the abdomen through the esophageal hiatus of the diaphragm, and terminates in the fundus of the stomach. Its muscle fibers originate from the cricoid cartilage and pharynx above and interdigitate with those of the stomach below. About 2-4 cm of the esophagus is normally located below the diaphragm. The junction between the esophagus and stomach is maintained in its normal intra-abdominal position by the reflection of the peritoneum onto the stomach and of the phrenoesophageal ligament onto the esophagus. The latter is a fibroelastic membrane that lies beneath the peritoneum, on the inferior surface of the diaphragm. When it reaches the esophageal hiatus, the ligament is reflected in an orad direction onto the lower esophagus, where it inserts into the circular muscle layer above the gastroesophageal sphincter, 2-4 cm above the diaphragm.
Three anatomic areas of narrowing occur in the esophagus: (1) at the level of the cricoid cartilage (pharyngoesophageal or upper esophageal sphincter [UES]); (2) in the mid thorax, from compression by the aortic arch and the left main stem bronchus; and (3) at the level of the esophageal hiatus of the diaphragm (gastroesophageal or lower esophageal sphincter [LES]).
In the adult, the distance as measured from the upper incisor teeth to the cricopharyngeus muscle is 15-20 cm; to the aortic arch, 20-25 cm; to the inferior pulmonary vein, 30-35 cm; and to the gastroesophageal junction, approximately 40-45 cm.
The mucosal lining of the esophagus consists of stratified squamous epithelium that contains scattered mucous glands throughout. The musculature of the pharynx and upper third of the esophagus is skeletal in type (striated muscle); the remainder is smooth muscle. Physiologically, the entire organ behaves as a single functioning unit, so that no distinction can be made between the upper and lower esophagus from the standpoint of propulsive activity. As in the intestinal tract, the muscle fibers are arranged into inner circular and outer longitudinal layers. The esophagus has no serosal layer. The arterial supply to the esophagus is quite consistent. The upper end is supplied by branches from the inferior thyroid arteries. The thoracic portion receives blood from the bronchial arteries and from esophageal branches originating directly from the aorta. The intercostal arteries may also contribute. The diaphragmatic and abdominal segments are nourished by the left inferior phrenic artery and by the esophageal branches of the left gastric artery. The venous drainage is more complex and variable. The most important veins are those that drain the lower esophagus. Blood from this region passes into the esophageal branches of the coronary vein, a tributary of the portal vein. This connection constitutes a direct communication between the portal circulation and the venous drainage of the lower esophagus and upper stomach. When there is portal hypertension, as in cirrhosis of the liver, blood is shunted upward through the coronary vein and the esophageal venous plexus to eventually pass by way of the azygos vein into the superior vena cava. The esophageal veins may eventually form varices as they become distended when portal hypertension is present.
The coordinated activity of the UES, the esophageal body and the LES is responsible for the motor function of the esophagus.
The UES receives motor innervation directly from the brain (nucleus ambiguous). The sphincter is continuously in a state of tonic contraction, with a resting pressure of about 100 mm Hg (anteroposterior axis). The sphincter prevents passage of air from the pharynx into the esophagus and reflux of esophageal contents into the pharynx. During swallowing, a food bolus is moved by the tongue into the pharynx, which contracts while the UES relaxes. After the food bolus has reached the esophagus, the UES regains its resting tone (Figure 20–2).
When food passes through the UES, a contraction is initiated in the upper esophagus, which progresses distally toward the stomach. The wave initiated by swallowing is referred as primary peristalsis (Figure 20–2). It travels at a speed of 3-4 cm/s and reaches amplitudes of 60-140 mm Hg in the distal esophagus. Local stimulation by distention at any point in the body of the esophagus will elicit a peristaltic wave from the point of stimulus. This is called secondary peristalsis and aids esophageal emptying when the primary wave has failed to clear the lumen of ingested food, or when gastric contents reflux from the stomach. Tertiary waves are considered abnormal, but they are frequently seen in elderly subjects who have no symptoms of esophageal disease.
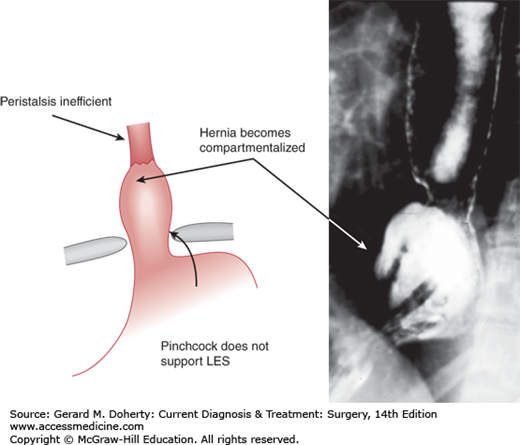
The LES measures 3-4 cm in length and its resting pressure ranges between 15 and 24 mm Hg. At the time of swallowing, the LES relaxes for 5-10 seconds to allow the food bolus to enter the stomach and then regains its resting tone (Figure 20–2). The LES relaxation is mediated by vasoactive intestinal polypeptide and nitric oxide, both nonadrenergic, noncholinergic neurotransmitters. The resting tone depends mainly on intrinsic myogenic activity. The LES has a tendency to relax periodically at times independent from swallowing. These periodic relaxations are called transient lower esophageal sphincter relaxations to distinguish them from relaxations triggered by swallows. The cause of these transient relaxations is not known, but gastric distention probably plays a role. Transient LES relaxations account for the small amount of physiologic gastroesophageal reflux present in any individual, and are also the most common cause of reflux in patients with gastroesophageal reflux disease (GERD). Decrease in length or pressure of the LES (or both) is responsible for abnormal reflux in the remaining patients. Overall, it is thought that while transient LES relaxation is the most common mechanism of reflux in volunteers and patients with either absent or mild esophagitis, the prevalence of a mechanically defective sphincter (hypotensive and short) increases in patients with severe esophagitis, particularly when Barrett metaplasia is present. The crus of the esophageal hiatus of the diaphragm contributes to the resting pressure of the LES. This pinchcock action of the diaphragm is particularly important because it protects against reflux caused by sudden increases of intra-abdominal pressure, such as with coughing or bending. This synergistic action of the diaphragm is lost when a sliding hiatal hernia is present, as the gastroesophageal junction is displaced above the diaphragm (Figure 20–3).
Esophageal symptoms can be divided into two groups: (1) typical, such as dysphagia, heartburn, and regurgitation and (2) atypical such as chest pain, cough, and hoarseness (Table 20–1). Dysphagia is a unique symptom as it points to an esophageal disorder, either functional (secondary to abnormalities of esophageal peristalsis or lack of coordination between different parts of the esophagus) or mechanical (secondary to a peptic or malignant stricture or an intraluminal mass). Heartburn can also be caused by non-esophageal disorders such as biliary disease, irritable bowel syndrome, coronary artery disease, and psychiatric diseases.
The upper gastrointestinal series test is performed by giving the patient barium to swallow. Subsequently, multiple images are taken, including the esophagus, the gastroesophageal junction, the stomach, and the duodenum (a barium swallow focuses just on the esophagus and the gastroesophageal junction). This test characterizes a hiatal hernia, an esophageal stricture, an esophageal diverticulum, or an intraluminal mass. A cine-esophagram is instead a dynamic evaluation of the swallowing process, and it is particularly useful in patients with functional dysphagia (secondary to a motility disorder, in the absence of a mechanical cause).
This test allows visualization of the mucosal surface of the esophagus, the stomach, and the duodenum. The presence and degree of esophagitis and the presence of an intraluminal mass can be determined, and biopsies taken.
This test is used in patients with esophageal cancer to define the depth of penetration of the tumor through the esophageal wall (T) and the presence of enlarged periesophageal lymph nodes (N). Fine-needle aspiration of these nodes can be done, and cytologic analysis of the aspirate performed.
Esophageal manometry allows determination of: (1) LES location, length, pressure, and relaxation in response to swallowing; (2) pressure, duration, and velocity of propagation of the peristaltic waves; and (3) location, pressure, relaxation of the UES, and coordination with the pharyngeal contraction.
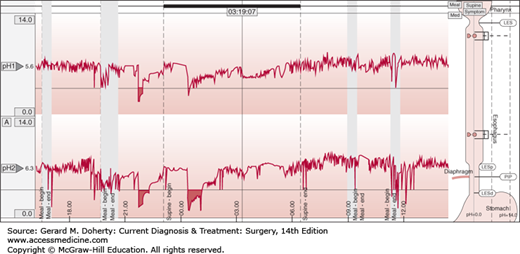
This test measures reflux of acid from the stomach into the esophagus, and it is considered the gold standard for the diagnosis of GERD. By convention, the catheter is placed 5 cm above the upper border of the manometrically determined LES and is kept in place for 24 hours, during which the patient does not alter the daily activities and diet. In addition to defining whether a pathologic amount of GER is present, the test establishes if there is a temporal correlation between episodes of reflux and symptoms (Figure 20–4). Esophageal impedance is a technique that measures flow of liquids and gas across the gastroesophageal junction, independently of the pH of the gastric refluxate. In association with pH monitoring, impedance is indicated in patients with proton pump inhibitors (PPIs)—resistant typical reflux symptoms and chronic unexplained cough.
Figure 20–4.
Ambulatory pH monitoring. The superior plot reports the 24-hour pH recording in the proximal esophagus 20 cm above the upper border of the manometrically determined LES. The inferior plot reports the 24-hour pH recording in the distal esophagus 5 cm above the upper border of the manometrically determined LES. A reflux episode occurs when pH falls below pH = 4 and ends when pH returns above 4.
It is indicated in patients who experience postprandial bloating and fullness to measure the gastric emptying of solids and liquids.
A computerized tomography (CT) scan is used to assess the presence of metastases (lung, liver, adrenals) in patients with esophageal cancer (M).
A positron emission tomography (PET) scan is used to assess the metastatic spread of esophageal cancer (M). In addition, it might help predict the response of esophageal cancer to neoadjuvant therapy.
Laparoscopy or thoracoscopy can be used to stage esophageal cancer, particularly when liver metastases or extensive lymphadenopathy are suspected.
ESOPHAGEAL MOTILITY DISORDERS
The named primary esophageal motility disorders are achalasia, diffuse esophageal spasm, nutcracker esophagus, and the hypertensive LES. They occur in the absence of any other esophageal disorder such as reflux, and their cause is unknown. These disorders present with a combination of dysphagia, regurgitation, chest pain, and heartburn. Esophageal manometry is the key test that differentiates these disorders.
ESSENTIALS OF DIAGNOSIS
Dysphagia
Regurgitation
Absence of esophageal peristalsis on esophageal manometry
Radiologic evidence of distal esophageal narrowing
Esophageal achalasia is characterized by the absence of esophageal peristalsis. In most patients, the LES is hypertensive and fails to relax appropriately in response to swallowing. These abnormalities lead to impaired emptying of food with consequent stasis in the esophagus. The incidence of achalasia is about 1 in 100,000 persons. It affects men more than women, and it can occur at any age.
The etiology is still unknown, but two theories exist: (1) a degenerative disease of the neurons; and (2) infections of the neurons by a virus (eg, herpes zoster) or another infectious agent. The latter is supported by the fact that similar findings occur in patients with Chagas disease (American trypanosomiasis), a condition in which the infective organism destroys parasympathetic ganglion cells throughout the body, including the heart and the gastrointestinal, urinary, and respiratory tracts. The degeneration of the myenteric plexus of Auerbach determines loss of the postganglionic inhibitory neurons (which contain nitric oxide and vasoactive intestinal polypeptide), which mediate LES relaxation. Because the postganglionic cholinergic neurons are spared, there is unopposed cholinergic stimulation, which increases LES resting pressure and decreases LES relaxation. There is no propagation of peristaltic waves in response to swallowing, but rather the presence of simultaneous contractions.
Dysphagia is the most common symptom, experienced by about 95% of patients. It is often for both solids and liquids. Most patients adapt with changes in their diet and are able to maintain a stable weight, while others eventually experience some weight loss. Regurgitation of undigested food is the second-most common symptom and it is present in about 60% of patients. It occurs more often in the supine position and may lead to aspiration. Heartburn is present in about 40% of patients. It is not due to GER, but rather to stasis and fermentation of undigested food in the distal esophagus. Chest pain also occurs in about 40% of patients, due to esophageal distension, and it is usually experienced at the time of a meal.
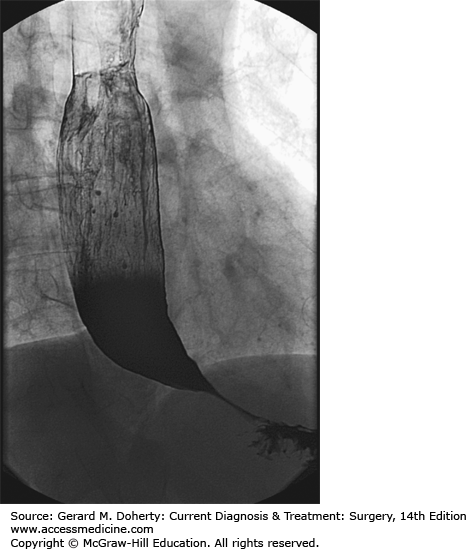
Endoscopy is usually the first test performed to rule out a mechanical obstruction such as a peptic stricture or cancer. A barium swallow usually shows narrowing at the level of the gastroesophageal junction, and slow emptying of contrast (Figure 20–5). A dilated, sigmoid esophagus may be present in patients with longstanding achalasia.
Esophageal manometry is the gold standard for establishing the diagnosis of esophageal achalasia. The classic manometric findings are: (1) absence of esophageal peristalsis and (2) hypertensive LES (in about 50% of patients) that relaxes only partially in response to swallowing. When the esophagus is dilated and sigmoid in shape, it may be difficult to pass the catheter through the gastroesophageal junction into the stomach. In these cases, the catheter may be placed under fluoroscopic or endoscopic guidance. Recently, a new classification of esophageal achalasia has been proposed based on high-resolution manometry: type I, classic, with minimal esophageal pressurization; type II, achalasia with esophageal compression; and type III, achalasia with spasm.
Benign strictures due to GER and esophageal carcinoma may mimic the clinical presentation of achalasia. Sometimes an infiltrating tumor of the gastroesophageal junction can mimic not only the clinical and radiological presentation of achalasia but also the manometric profile. This condition, called secondary or pseudo-achalasia, should be suspected in patients older than 60 years with recent onset of dysphagia (< 6 months) and excessive weight loss. An endoscopic ultrasound or a CT scan with fine cuts is recommended to rule out an underlying malignancy.
Aspiration of retained and undigested food can cause repeated episodes of pneumonia. Achalasia is also a risk factor for esophageal squamous cell carcinoma, probably due to the continuous irritation of the mucosa by the retained and fermenting food. Adenocarcinoma can occur in patients who develop GER after either pneumatic dilation or myotomy.
Therapy is palliative, and it is directed toward relief of symptoms by decreasing the outflow resistance caused by the dysfunctional LES. Because peristalsis is absent and does not return after any form of treatment, gravity becomes the key factor that allows emptying of food from the esophagus into the stomach.
Calcium-channel blockers are used to decrease LES pressure. However, because only 10% of patients benefit from this treatment, it should be used primarily in patients who have contraindications to either pneumatic dilation or to surgery.
Intrasphincteric injection of botulinum toxin is used to block the release of acetylcholine at the level of the LES, thereby restoring the balance between excitatory and inhibitory neurotransmitters. This treatment, however, is of limited value. Only 60% of treated patients still have relief of dysphagia 6 months after treatment, and this number further decreases to 30% (even after multiple injections) 2.5 years later. In addition, it often causes an inflammatory reaction at the level of the gastroesophageal junction, which makes a subsequent myotomy more difficult. It should be used primarily in patients who are poor candidates for dilatation or surgery.
Pneumatic dilation of the LES is considered the most effective nonsurgical treatment of achalasia and has been the main modality of treatment for many years until the advent of minimally invasive surgery in the early 1990s. A balloon is inflated at the level of the gastroesophageal junction to rupture the muscle fibers while trying to leave the mucosa intact. The initial success rate is around 90%, but it decreases in most patients to 50% at 10 years, even after multiple dilations. The perforation rate is about 2%-5%. If a free perforation occurs, patients are taken emergently to the operating room, where closure of the perforation and a myotomy on the contralateral side of the esophagus are performed. The incidence of post-dilation GER is about 25%-35%. Patients who fail pneumatic dilation are usually treated by a laparoscopic Heller myotomy.
A recent novel approach to achalasia is the per-oral endoscopic esophageal myotomy (POEM). During this procedure the circular muscle fibers of the lower esophagus and the upper stomach are cut through a sub-mucosal tunnel. Long-term follow-up will be needed to assess the long-term results of this procedure.
A laparoscopic Heller myotomy and partial fundoplication has progressively become the procedure of choice for esophageal achalasia during the last 20 years. The operation consists of a controlled section of the muscle fibers (myotomy) of the lower esophagus (6 cm) and proximal gastric wall (2-2.5 cm), followed by an anterior or a posterior partial fundoplication to prevent reflux. Patients spend 24-48 hours in the hospital, and return to regular activities in about 2 weeks. The operation effectively relieves symptoms in about 90% of patients, and it is effective even in patients who have a low LES pressure after previous dilation or when the esophagus is dilated. Therefore, it should be preferred to pneumatic dilation whenever surgical expertise is available. The incidence of postoperative reflux is around 25%-35%, and it is usually controlled by acid reducing medications. Persistent or recurrent dysphagia after myotomy can be treated with pneumatic dilation or another myotomy. Esophagectomy is reserved for patients with severe dysphagia who have failed both dilation and myotomy.
ESSENTIALS OF DIAGNOSIS
Dysphagia
Chest pain
Intermittent symptoms
Radiologic evidence of tertiary contractions (corkscrew esophagus)
Intermittent normal and absent peristaltic waves on manometry (> 10%, < 100%)
Normal 24-h ambulatory pH monitoring
The cause of diffuse esophageal spasm is not known. Stress might play a role. Progression of diffuse esophageal spasm to achalasia has been documented.
The most common symptom is intermittent chest pain, which varies from slight discomfort to severe spasmodic pain that simulates the pain of coronary artery disease. Most patients complain of dysphagia, but weight loss is uncommon.
The barium swallow is abnormal in about 70% of patients. Fluoroscopic studies show segmental spasms, areas of narrowing, and irregular uncoordinated peristalsis (corkscrew esophagus) in about 30% of patients. An epiphrenic diverticulum is sometimes present.
Esophageal manometry is the key test for establishing the diagnosis of diffuse esophageal spasm. The classic manometric findings are: (1) alternation of esophageal peristalsis and simultaneous contractions (> 10% and < 100%) and (2) normal LES function or abnormalities similar to those seen in achalasia.
This test is essential as the symptoms and the manometric picture of diffuse esophageal spasm can be caused by GERD. In such cases, treatment should be directed toward reflux because the dysmotility is secondary to the reflux. Therefore, it is crucial to be certain about the diagnosis, as treatment of GERD (acid-reducing medications or a fundoplication) is completely different from that of a primary esophageal motility disorder (pneumatic dilation or myotomy).
When chest pain is the predominant symptom, a complete cardiac workup is necessary to exclude a cardiac reason for the pain. Once the heart disease has been excluded, ambulatory pH monitoring must be performed to rule out abnormal GER, which is the most common cause of noncardiac chest pain. Esophageal manometry is the only test that distinguishes diffuse esophageal spasm from other primary esophageal motor disorders. An endoscopy should be performed to confirm the absence of intraluminal lesions.
Regurgitation and aspiration may occur, possibly leading to repeated episodes of pneumonia. An epiphrenic diverticulum may be present, secondary to the motor disorder.
The therapeutic approach to diffuse esophageal spasm is similar to that of achalasia. Both disorders can be conceptualized as different points in a spectrum of esophageal motility, where peristalsis is progressively lost. In patients with diffuse esophageal spasm, dysphagia is secondary to abnormalities of the peristalsis and the LES, while the chest pain probably results from esophageal distension from poor emptying. Medical therapy (long-acting nitrates, calcium-channel blocking agents) is relatively ineffective. Pneumatic dilation improves the dysphagia in about 25% of patients. Intrasphincteric injection of botulinum toxin has also given poor results. In contrast, a laparoscopic Heller myotomy and partial fundoplication (as for patients with achalasia) improves both dysphagia and chest pain in about 80% of patients.
The hypertensive lower esophageal sphincter is a rare disorder that manifests with dysphagia and is characterized manometrically by a hypertensive LES (resting pressure > 45 mm Hg), which relaxes in response to swallowing, and normal esophageal peristalsis. Treatment is similar to that of esophageal achalasia.
ESSENTIALS OF DIAGNOSIS
Chest pain
Dysphagia
Intermittent symptoms
Peristaltic waves propagate normally but have very high amplitude and long duration
Normal 24-h ambulatory pH monitoring
The cause of this disorder is not known.
Chest pain is the most common symptom. Patients often come to the attention of gastroenterologists only after a thorough cardiac workup has been performed. About half of the patients complain of dysphagia in addition to chest pain.
The barium swallow is usually normal. An epiphrenic diverticulum is sometimes present.
Esophageal manometry is the key test for establishing the diagnosis of nutcracker esophagus. The classic manometric findings are as follows: (1) normal propagation of the peristalsis waves (there are no simultaneous contractions)—the peristaltic waves in the distal esophagus, however, have very high amplitude (> 180 mm Hg) and duration (> 6 sec) and (2) normal LES function or abnormalities similar to those seen in achalasia and diffuse esophageal spasm.
This test is essential because the symptoms and the manometric picture of nutcracker esophagus can be caused by GERD. In such cases, treatment should be directed toward reflux because the dysmotility is secondary.
When chest pain is the predominant symptom, a complete cardiac work up is necessary to exclude a cardiac reason for the pain. Once the heart has been excluded as a cause of the symptom, ambulatory pH monitoring must be performed to rule out abnormal GER, which is the most common cause of noncardiac chest pain. Esophageal manometry is the only test that distinguishes nutcracker esophagus from other primary esophageal motility disorders.
Regurgitation and aspiration may occur, possibly leading to repeated pneumonic infections. An epiphrenic diverticulum may be present, secondary to the motor disorder.
The nutcracker esophagus is not as well defined as the other primary esophageal motility disorders for both pathophysiology and treatment. Initially, it was thought that the high pressure of the peristaltic contractions was the cause of the chest pain, so treatment was aimed at decreasing the high amplitude of the peristaltic waves. However, calcium-channel blockers are unable to improve the chest pain even though they decrease the strength of the contractions. Similarly, the results of surgery have been disappointing, as chest pain persists after myotomy in about 50% of patients. Dysphagia is improved in 80% of patients.
Esophageal diverticula are rare. They are located above the UES (pharyngoesophageal or Zenker diverticulum) or the LES (epiphrenic diverticulum). They are considered pulsion diverticula and are secondary to abnormalities of the sphincters in terms of resting pressure, relaxation in response to swallowing, and coordination with the segment above the sphincter. As a consequence, mucosa and submucosa protrude through the muscular layers, forming the outpouching.
ESSENTIALS OF DIAGNOSIS
Dysphagia
Regurgitation of undigested food (with risk of aspiration)
Gurgling sounds in the neck
Halitosis
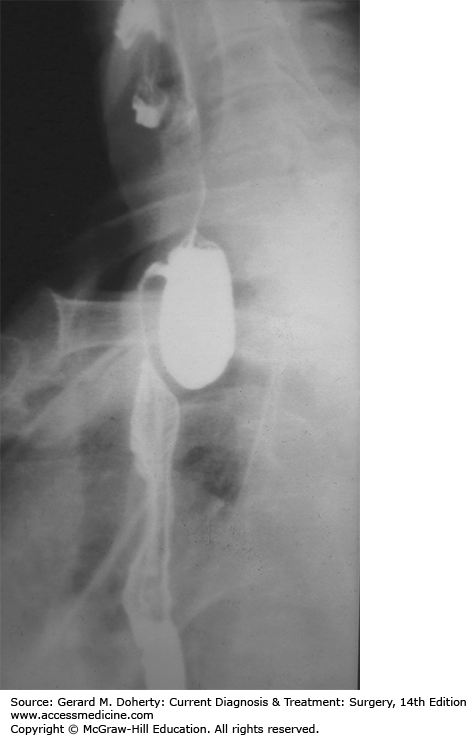
This is the most common of the esophageal diverticula and is three times more frequent in men than in women. Most patients are over age 60 years. The condition originates from the posterior wall of the esophagus, in a triangular area of weakness (Killian triangle), limited inferiorly by the upper border of the cricopharyngeal muscle and laterally by the oblique fibers of the inferior constrictor muscles of the pharynx. As the diverticulum enlarges, it tends to deviate from the midline, mostly to the left.
A Zenker diverticulum is due either to lack of coordination between the pharyngeal contraction and the opening time of the UES or to a hypertensive UES. Because of the increased intraluminal pressure, there is progressive herniation of mucosa and submucosa through the Killian triangle. Occasionally, UES dysfunction can occur in the absence of a diverticulum (cricopharyngeal achalasia). A hereditary syndrome called oculopharyngeal muscular dystrophy, consisting of ptosis and dysphagia, has been described in patients of French-Canadian ancestry. The dysphagia is the result of weak pharyngeal musculature in the face of normal UES function; it is considerably improved by UES myotomy. This syndrome also manifests with cervical dysphagia. A chronic cough may develop in some patients from aspiration of saliva and ingested food.
Dysphagia is the most common symptom and occurs in about 80%-90% of patients. Regurgitation of undigested food from the diverticulum often occurs, and can lead to aspiration into the tracheobronchial tree and pneumonia. Patients frequently have halitosis and can hear gurgling sounds in the neck. About 30% of patients have associated GERD.
A barium swallow clearly shows the position and size of the diverticulum or a prominent cricopharyngeal bar without diverticulum (Figure 20–6).
Esophageal manometry shows a lack of coordination between the pharynx and the cricopharyngeus muscle and often a hypertensive UES. In addition, it can show a hypotensive LES and abnormal esophageal peristalsis. Ambulatory pH monitoring determines if abnormal esophageal acid exposure is present. Endoscopy may be dangerous because the instrument can enter the diverticulum rather than the esophageal lumen and cause a perforation.
Differential diagnosis includes esophageal stricture, achalasia, and esophageal cancer.
The standard treatment consists of eliminating the functional obstruction at the UES level (myotomy of the cricopharyngeus muscle and the upper 3 cm of the posterior esophageal wall) and excision or suspension of the diverticulum. For small diverticula (< 2 cm), the myotomy alone is sufficient. As an alternative to the conventional surgical treatment, a transoral endoscopic approach (using staplers, laser or coagulation through an endoscope that ablate the septum between the diverticulum and the cervical esophagus) can be used for diverticula between 3 and 6 cm in size. If present, GER should be corrected before dividing the UES in order to avoid aspiration. The prognosis is excellent in about 90% of cases. Complications are rare and the patients are usually able to eat the day after the procedure.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree