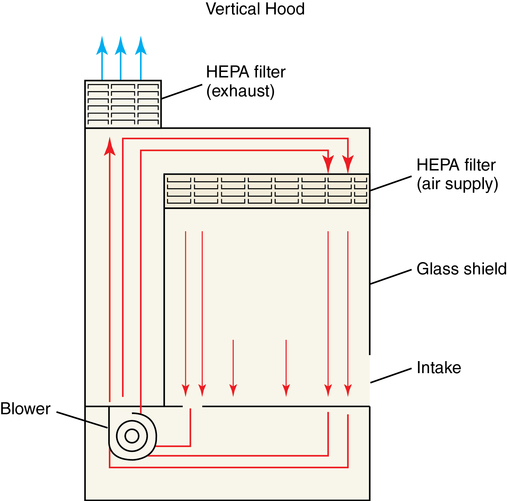
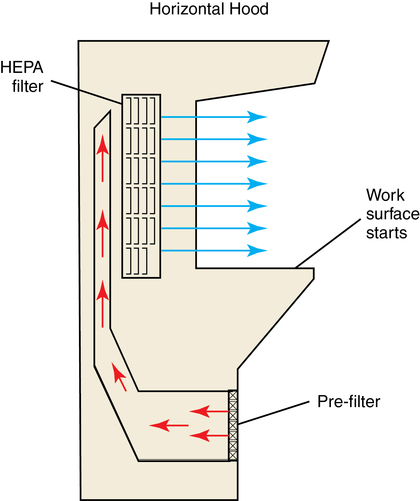
1. Discuss USP 797 guidelines and their importance in preventing microbial contamination. 2. Discuss requirements for the intravenous area according to USP 797, and demonstrate environmental procedures, including laminar air flow hood cleaning and proper garbing techniques. 3. Identify supplies used in aseptic technique, including how to read syringes. Biological safety cabinet (BSC) Special hood where air flows downward through a HEPA filter; used for chemotherapy preparation ISO Class 5 area where LAFW or other PECs are physically located and aseptic manipulations occur Term sometimes used for buffer area ISO Class 5 environment where aseptic manipulations take place Area within the ISO Class 5 primary engineering control where manipulations are performed Apparel or clothing that should be worn during aseptic preparation Size of the needle shaft (thickness); the finer the needle, the higher the gauge number High-efficiency particulate air (HEPA) filter Laminar airflow workbench (LAFW) Containers of sterile solution used for intravenous medications; usually 500 mL to 3000 mL in volume Personal protective equipment (PPE) Equipment, including shoe and hair covers, beard covers, gowns, masks, and gloves Primary engineering control (PEC) Controls such as LAFWs, compounding aseptic isolators, or BSCs located in the buffer area The first step in preparing aseptic preparations is the environment itself. The space where sterile preparation takes place must be clean and free from contaminates. This working space is divided into three sections (Figure 6-1). The LAFW or BSC are both areas that provide a Class 5 environment for aseptic preparation inside the buffer area (Figure 6-2). Sitting in front of the LAFW is like sitting in front of a fan blowing wind in your face. The air, known as critical air, enters the prefilter at the front of the hood, travels through the HEPA filter at the back where bacteria and other air contaminants are removed, and then flows horizontally across the work surface (Figure 6-3). This allows purified air to circulate from the back to the front constantly in parallel lines. The air space inside the LAFW is the area known as the DCA. This is where exposure to HEPA-filtered air, or first air, occurs when preparing aseptic preparations. Critical sites (such as the tops of vials and needle surfaces) should always be exposed to first air to avoid contamination from particles allowed to linger in the air. Surfaces (such as needles, syringe plungers, or vial tops) should be exposed to first air at all times. Observe the following guidelines when working in the LAFW: • Always perform all manipulations at least 6 inches inside the hood. • Remember first air: Never interrupt the airflow between the HEPA filter and sterile objects. • Avoid spraying or wiping the HEPA filter. • The hood should be turned on at least 30 minutes prior to using. • Always clean the LAFW with an approved agent (70% isopropyl alcohol [IPA]) and lint-free wipes.
Equipment and facilities
Introduction
Environment, garbing, and equipment cleaning procedures
Laminar airflow workbench and biological safety cabinet
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Equipment and facilities