Epilepsy
KEY CONCEPTS
![]() Patient-specific treatment goals should be identified as early as possible.
Patient-specific treatment goals should be identified as early as possible.
![]() Accurate diagnosis and classification of seizure/syndrome type is critical to selection of appropriate pharmacotherapy.
Accurate diagnosis and classification of seizure/syndrome type is critical to selection of appropriate pharmacotherapy.
![]() Patient characteristics such as age, comorbid conditions, ability to comply with the prescribed regimen, and presence or absence of insurance coverage can also influence the choice of antiepileptic drugs (AEDs).
Patient characteristics such as age, comorbid conditions, ability to comply with the prescribed regimen, and presence or absence of insurance coverage can also influence the choice of antiepileptic drugs (AEDs).
![]() Pharmacotherapy of epilepsy is highly individualized and requires titration of the dose to optimize AED therapy (maximal seizure control with minimal or no side effects). Approximately 50% to 70% of patients can be maintained on one AED.
Pharmacotherapy of epilepsy is highly individualized and requires titration of the dose to optimize AED therapy (maximal seizure control with minimal or no side effects). Approximately 50% to 70% of patients can be maintained on one AED.
![]() If the therapeutic goal is not achieved with monotherapy, a second drug can be added or a switch to an alternative single AED can be made. If a second AED is added it should have a different mechanism of action from the first, although there is no clear evidence in humans to support this.
If the therapeutic goal is not achieved with monotherapy, a second drug can be added or a switch to an alternative single AED can be made. If a second AED is added it should have a different mechanism of action from the first, although there is no clear evidence in humans to support this.
![]() Some patients eventually can discontinue AED therapy. Several factors predict successful withdrawal of AEDs.
Some patients eventually can discontinue AED therapy. Several factors predict successful withdrawal of AEDs.
![]() Surgery is the treatment of choice in selected patients with refractory focal epilepsy.
Surgery is the treatment of choice in selected patients with refractory focal epilepsy.
![]() The appropriate use of AEDs requires a thorough understanding of their clinical pharmacology, including mechanism of action, pharmacokinetics, adverse reactions, and drug interactions, as well as available dosage forms.
The appropriate use of AEDs requires a thorough understanding of their clinical pharmacology, including mechanism of action, pharmacokinetics, adverse reactions, and drug interactions, as well as available dosage forms.
Epilepsy is a disorder that is best viewed as a symptom of disturbed electrical activity in the brain, which may have many etiologies. It is a collection of many different types of seizures that vary widely in severity, appearance, cause, consequence, and management. Seizures that are prolonged or repetitive can be life-threatening. Epilepsy is defined by the occurrence of at least two unprovoked seizures separated by 24 hours.1 The effect epilepsy has on patients’ lives can be significant and extremely frustrating. It is also important to recognize that seizures can be just one (albeit the most obvious) symptom of an epileptic disorder. Not uncommonly, patients have other comorbid disorders, including depression, anxiety, and potentially neuroendocrine disturbances. Patients with epilepsy also may display neurodevelopmental delay, memory problems, and/or cognitive impairment. Although, by convention, the focus of drug treatment is on the abolition of seizures, clinicians must also try to address these common comorbidities.
EPIDEMIOLOGY
Each year, 120 per 100,000 people in the United States come to medical attention because of a newly recognized seizure.1 At least 8% of the general population will have at least one seizure in a lifetime. However, it is common to have a seizure and not have epilepsy. The rate of recurrence of a first unprovoked seizure within 5 years ranges between 23% and 80%. Children with an idiopathic first seizure and a normal electroencephalogram (EEG) have a particularly favorable prognosis. Some seizures occur as single events resulting from withdrawal of CNS depressants (e.g., alcohol, barbiturates, and other drugs) or during acute neurologic illnesses or systemic toxic conditions (e.g., uremia or eclampsia). Some patients will have seizures only associated with fever. These febrile seizures do not constitute epilepsy.1
The age-adjusted incidence of epilepsy is 44 per 100,000 person-years. Each year, approximately 125,000 new epilepsy cases occur in the United States; only 30% are in people younger than 18 years of age at the time of diagnosis. There is a bimodal distribution in the occurrence of the first seizure, with one peak occurring in newborn and young children and the second peak occurring in patients older than 65 years of age. The relatively high frequency of epilepsy in the elderly is now being recognized.
ETIOLOGY
Seizures occur because a group of cortical neurons discharge abnormally in synchrony. Anything that disrupts the normal homeostasis or stability of neurons can trigger hyperexcitability and seizures. Thousands of medical conditions can cause epilepsy, from genetic mutations to traumatic brain injury. A genetic predisposition to seizures has been observed in many forms of primary generalized epilepsy. Patients with mental retardation, cerebral palsy, head injury, or strokes are at an increased risk for seizures and epilepsy. The more profound the degree of mental retardation as measured by the intelligence quotient (IQ), the greater is the incidence of epilepsy. In the elderly, the onset of seizures is typically associated with focal neuronal injury induced by strokes, neurodegenerative disorders (e.g., Alzheimer’s disease), and other conditions. In some cases, if an etiology of seizures can be identified and corrected, the patient may not require chronic antiepileptic drug (AED) treatment. Patients can also present with unprovoked seizures that do not have an identifiable cause, and thus by definition have idiopathic or cryptogenic epilepsy. Idiopathic etiology is the term used for suspected genetic cause, whereas cryptogenic etiology is used if no obvious cause is found for focal-onset seizures.
Many factors have been shown to precipitate seizures in susceptible individuals. Hyperventilation can precipitate absence seizures. Excessive sleep, sleep deprivation, sensory stimuli, emotional stress, and hormonal changes occurring around the time of menses, puberty, or pregnancy have been associated with the onset of or an increased frequency of seizures. A careful drug history should be obtained from patients presenting with seizures because theophylline, alcohol, high-dose phenothiazines, antidepressants (especially maprotiline or bupropion), and street drug use have been associated with provoking seizures. Perinatal injuries and small gestational weight at birth are also risk factors for the development of partial-onset seizures. Immunizations have not been associated with an increased risk of epilepsy.
PATHOPHYSIOLOGY
Seizures result from excessive excitation, or in the case of absence seizures, from disordered inhibition of a large population of cortical neurons.2 This is reflected on EEG as a sharp wave or spike. Initially, a small number of neurons fire abnormally. Normal membrane conductances and inhibitory synaptic currents break down, and excess excitability spreads, either locally to produce a focal seizure or more widely to produce a generalized seizure. This onset propagates by physiologic pathways to involve adjacent or remote areas. The clinical manifestations depend on the site of the focus, the degree of irritability of the surrounding area of the brain, and the intensity of the impulse.2
There are multiple mechanisms that might contribute to synchronous hyperexcitability, including: (a) alterations in the distribution, number, type, and biophysical properties of ion channels in the neuronal membranes; (b) biochemical modifications of receptors; (c) modulation of second messaging systems and gene expression; (d) changes in extracellular ion concentrations; (e) alterations in neurotransmitter uptake and metabolism in glial cells; and (f) modifications in the ratio and function of inhibitory circuits. In addition, local neurotransmitter imbalances could be a potential mechanism for focal epileptogenesis. Transitory imbalances between the main neurotransmitters, glutamate (excitatory) and γ-aminobutyric acid (GABA) (inhibitory), and neuromodulators (e.g., acetylcholine, norepinephrine, and serotonin) might play a role in precipitating seizures in susceptible patients.2
Control of abnormal neuronal activity with AEDs is accomplished by elevating the threshold of neurons to electrical or chemical stimuli or by limiting the propagation of the seizure discharge from its origin. Raising the threshold most likely involves stabilization of neuronal membranes, whereas limiting the propagation involves depression of synaptic transmission and reduction of nerve conduction.2
Prolonged seizures and continued exposure to glutamate can result in neuronal injury in vulnerable neuronal populations resulting in functional deficits, primarily in memory, and in permanent changes of wiring of the neuronal circuitry. Sprouting and reorganization of neuronal projections might lead to a chronic susceptibility to seizures, neuronal destruction, and brain damage. However, limited degree of neurogenesis in the hippocampal pathways has been induced by epileptic seizures. The role of these newly born neurons is not well understood.
CLINICAL PRESENTATION
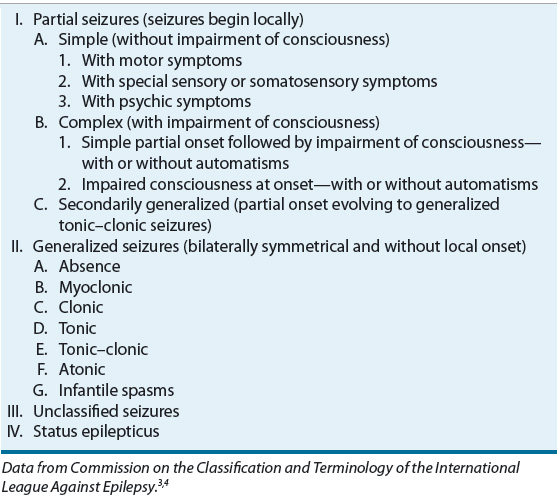
The International League Against Epilepsy has proposed two major schemes for the classification of seizures and epilepsies: the International Classification of Epileptic Seizures and the International Classification of the Epilepsies and Epilepsy Syndromes.3,4 The International Classification of Epileptic Seizures (Table 40-1) combines the clinical description with certain electrophysiologic findings to classify epileptic seizures. Seizures are divided into two main pathophysiologic groups—partial seizures and generalized seizures—by EEG recordings and clinical symptomatology.
TABLE 40-1 International Classification of Epileptic Seizures
CLINICAL PRESENTATION Epilepsy
Partial (focal) seizures begin in one hemisphere of the brain and—unless they become secondarily generalized—result in an asymmetric motor manifestation. Partial seizures manifest as alterations in motor functions, sensory or somatosensory symptoms, or automatisms. Partial seizures with no loss of consciousness are classified as simple partial (SP). In some cases, patients will describe somatosensory symptoms as a “warning” prior to the development of a generalized tonic–clonic (GTC) seizure. These warnings are, in fact, SP seizures and frequently are termed auras.
Partial seizures with an alteration of consciousness are described as complex partial (CP). With CP seizures, the patient can have automatisms, periods of memory loss, or aberrations of behavior. Some patients with CP epilepsy have been mistakenly diagnosed as having psychotic episodes. CP seizures also can progress to GTC seizures. Patients with CP seizures typically are amnestic to these events. A partial seizure that becomes generalized is referred to as a secondarily generalized seizure.
Generalized seizures have clinical manifestations that indicate involvement of both hemispheres. Motor manifestations are bilateral, and there is a loss of consciousness. Generalized seizures can be further subdivided by EEG and clinical manifestations. Generalized absence seizures are manifested by a sudden onset, interruption of ongoing activities, a blank stare, and possibly a brief upward rotation of the eyes. They generally occur in young children through adolescence. It is important to differentiate absence seizures from CP seizures.
With GTC seizures there is a sudden sharp tonic contraction of muscles followed by a period of rigidity and clonic movements. During the seizure, the patient may cry or moan, lose sphincter control, bite the tongue, or develop cyanosis. After the seizure, the patient may have altered consciousness, drowsiness, or confusion for a variable period of time (postictal period) and frequently goes into a deep sleep. Tonic and clonic seizures can also occur separately.
Brief shock-like muscular contractions of the face, trunk, and extremities are known as myoclonic jerks. They can be isolated events or rapidly repetitive. A sudden loss of muscle tone is known as an atonic seizure, which may present as a head drop, the dropping of a limb, or a slumping to the ground. These patients often wear protective head ware to prevent trauma.
The International Classification of Epilepsies and Epilepsy Syndromes adds components such as age of onset, intellectual development, findings on neurologic examination, and results of neuroimaging studies to define epilepsy syndromes more fully. Syndromes can include one or many different seizure types (e.g., Lennox–Gastaut syndrome). The syndromic approach includes seizure type(s) and possible etiologic classifications (e.g., idiopathic, symptomatic, or unknown). Idiopathic describes syndromes that are presumably genetic but also those in which no underlying etiology is documented or suspected. A family history of seizures is commonly present, and neurologic function is essentially normal except for the occurrence of seizures. Symptomatic cases involve evidence of brain damage or a known underlying cause. A cryptogenic syndrome is assumed to be symptomatic of an underlying condition that cannot be documented. Unknown or undetermined is used when no cause can be identified. This syndromic classification requires more information and is more important for prognostic determinations and response to treatment than for a classification based simply on seizure type.
TREATMENT
Desired Outcomes
![]() The ideal goal of treatment for epilepsy is complete elimination of seizures and no side effects with an optimal quality of life (QOL). Data from a large systematic review found that optimal QOL in epilepsy patients is defined by decreasing their seizure frequency and severity as well as addressing comorbid conditions, especially anxiety and depression.6 A large multicenter study found that in pharmacoresistant epilepsy patients, the adverse effects of their AEDs and depressive comorbidity were far more important in determining QOL than reducing the frequency of their seizures when seizure freedom cannot be obtained.7 In addition, other factors that can impact QOL in epilepsy patients include issues about driving, economic security, forming relationships, safety, social isolation, and social stigma.
The ideal goal of treatment for epilepsy is complete elimination of seizures and no side effects with an optimal quality of life (QOL). Data from a large systematic review found that optimal QOL in epilepsy patients is defined by decreasing their seizure frequency and severity as well as addressing comorbid conditions, especially anxiety and depression.6 A large multicenter study found that in pharmacoresistant epilepsy patients, the adverse effects of their AEDs and depressive comorbidity were far more important in determining QOL than reducing the frequency of their seizures when seizure freedom cannot be obtained.7 In addition, other factors that can impact QOL in epilepsy patients include issues about driving, economic security, forming relationships, safety, social isolation, and social stigma.
The American Academy of Neurology (AAN) has developed eight quality performance measures for the clinician that define a high quality of care of these patients.8 In a recent survey of practicing neurologists, poor performance was found on three of these eight—counseling patients about AED side effects, discussion about depression, and their knowledge about referral of the intractable epilepsy patient for surgery.9 Lastly in helping to address QOL in epilepsy patients, an international consensus group has recently developed evidenced-based and practice-based statements to provide guidance on the management of neuropsychiatric conditions associated with epilepsy including depression.10
Clinical Controversy…
General Approach to Treatment
![]() The general approach to treatment involves assessment of seizure type and frequency, identification of treatment goals, development of a care plan, and a plan for followup evaluation. During the assessment phase, it is critical to establish an accurate diagnosis of the seizure type and classification in order to select the appropriate initial AEDs. Patient-specific treatment goals must be identified, and these can change over time. Despite appropriate AED treatment, approximately 30% to 35% of patients are refractory to treatment. In this setting, seizure freedom may not be obtained, and more obtainable goals should be established (e.g., decrease in the number of seizures and minimized drug adverse effects).
The general approach to treatment involves assessment of seizure type and frequency, identification of treatment goals, development of a care plan, and a plan for followup evaluation. During the assessment phase, it is critical to establish an accurate diagnosis of the seizure type and classification in order to select the appropriate initial AEDs. Patient-specific treatment goals must be identified, and these can change over time. Despite appropriate AED treatment, approximately 30% to 35% of patients are refractory to treatment. In this setting, seizure freedom may not be obtained, and more obtainable goals should be established (e.g., decrease in the number of seizures and minimized drug adverse effects).
![]() Patient characteristics such as age, medical condition, ability to comply with a prescribed regimen, and insurance coverage also should be explored because these can influence AED choices or help to explain nonadherence to the regimen, a lack of response, or unexpected adverse effects.
Patient characteristics such as age, medical condition, ability to comply with a prescribed regimen, and insurance coverage also should be explored because these can influence AED choices or help to explain nonadherence to the regimen, a lack of response, or unexpected adverse effects.
Once the assessment is complete, for patients with new-onset seizures, the choice is whether to use drug therapy and, if so, which one. For a patient with long-standing epilepsy, adequacy of the current medication regimen must be evaluated. An AED should not be considered ineffective unless the patient has experienced unacceptable adverse effects with continued seizures.
![]() If a decision is made to start AED therapy, monotherapy is preferred, and approximately 50% to 70% of all patients with epilepsy can be maintained on one drug.11 However, many of these patients are not seizure free. The percentage of patients who are seizure free on one drug varies by seizure type. After 12 months of treatment, the percentage who are seizure free is highest for those who have only GTC seizures (48% to 55%), lowest for those who have only CP seizures (23% to 26%), and intermediate for those with mixed seizure types (25% to 32%).11 Combining AEDs with different mechanisms of action to achieve freedom from seizures may be advantageous, although this approach is as yet unproven. Approximately 65% of patients can be expected to be maintained on one AED and be considered well controlled, although not necessarily seizure free.
If a decision is made to start AED therapy, monotherapy is preferred, and approximately 50% to 70% of all patients with epilepsy can be maintained on one drug.11 However, many of these patients are not seizure free. The percentage of patients who are seizure free on one drug varies by seizure type. After 12 months of treatment, the percentage who are seizure free is highest for those who have only GTC seizures (48% to 55%), lowest for those who have only CP seizures (23% to 26%), and intermediate for those with mixed seizure types (25% to 32%).11 Combining AEDs with different mechanisms of action to achieve freedom from seizures may be advantageous, although this approach is as yet unproven. Approximately 65% of patients can be expected to be maintained on one AED and be considered well controlled, although not necessarily seizure free.
![]() Of the 35% of patients with unsatisfactory control, 10% will be well controlled with a two-drug treatment. Of the remaining 25%, 20% will continue to have unsatisfactory control despite multiple drug treatment. There may be a genetic predisposition to epilepsy that is refractory to drug therapy. Some of these patients may become candidates for surgery or vagal nerve stimulator.
Of the 35% of patients with unsatisfactory control, 10% will be well controlled with a two-drug treatment. Of the remaining 25%, 20% will continue to have unsatisfactory control despite multiple drug treatment. There may be a genetic predisposition to epilepsy that is refractory to drug therapy. Some of these patients may become candidates for surgery or vagal nerve stimulator.
Once the care plan is established, an AED is selected. Patient education and assurance of patient understanding of the plan are essential. Detailed directions regarding titration, what to do in the event of a treatment-emergent side effect, and what to do if a seizure occurs must be provided to patients. Documentation of the assessment, care plan, and educational process is essential. Providing the patient with a seizure and side-effect diary will assist in the followup and evaluation phase. At the followup stage of treatment (which can be done in the hospital, clinic, pharmacy, or by phone), the treatment goals must be reviewed. If the goal has been achieved, new goals should be identified. For example, if the GTC seizures are now controlled, the goal may be to control partial seizures. If a patient fails to respond to the first AEDs, trials with other AEDs should be attempted as appropriate. Completion of the evaluation often requires a reassessment of the patient and development of a new care plan taking into account patient compliance, efficacy, and safety of the initial treatment.
Medication noncompliance can be the single most common reason for treatment failure. It is estimated that up to 60% of patients with epilepsy are noncompliant.12 The rate of noncompliance is increased by the complexity of the drug regimen and by doses taken three and four times a day. Frequent uncontrolled seizures can also predispose a patient to noncompliance secondary to confusion over whether the drug was taken. Noncompliance is not influenced by age, sex, psychomotor development, or seizure type.12
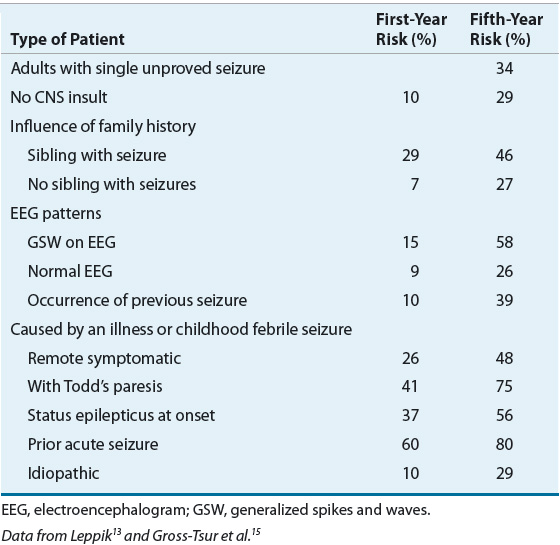
Difference of opinion exists on the most appropriate time to initiate AED therapy. Treatment decisions vary depending on individual patient clinical characteristics and circumstances. Some clinicians start AED treatment after the first seizure, whereas others do not initiate treatment until a second, unprovoked seizure has occurred. Still others initiate prophylactic treatment following a CNS insult thought likely to cause epilepsy eventually (e.g., stroke or head trauma). Drug treatment may not be indicated when seizures have minimal impact on patients’ lives or when there has been only a single seizure. If a patient presents after a single isolated seizure, one of three treatment decisions can be made: treat, possibly treat, or do not treat. These decisions are based on the probability of the patient having a second seizure (Table 40-2). For patients with no risk factors, the probability of a second seizure is less than 10% in the first year and approximately 24% by the end of 2 years. If risk factors are present, the recurrence rate can be as high as 80% after 5 years.13 The decision on whether to start AED therapy often depends on patient-specific factors such as epilepsy syndrome, seizure etiology, presence of a neuroanatomic defect, and the EEG, as well as, the patient’s lifestyle and preferences. Patients who have had two or more seizures generally should be started on AEDs.
TABLE 40-2 Recurrence Risk for Patients Experiencing One Unprovoked Seizure
When to Stop Antiepileptic Drugs
The AEDs used to control seizures may not need to be given for a lifetime. Polypharmacy can be reduced, and some patients can discontinue AEDs altogether. The drug considered less appropriate for the seizure type (or the agent deemed most responsible for adverse effects) should be discontinued first. In some cases, decreasing the number of AEDs can decrease side effects and increase cognitive abilities. This improvement in cognition may be small, especially if the patient is on a drug that primarily affects psychomotor speed with less effect on higher-order cognitive functioning.
![]() Factors favoring successful withdrawal of AEDs include a seizure-free period of 2 to 4 years, complete seizure control within 1 year of onset, an onset of seizures after age 2 but before age 35, and a normal neurologic examination and EEG. Factors associated with a poor prognosis in discontinuing AEDs, despite a seizure-free interval, include a history of a high frequency of seizures, repeated episodes of status epilepticus (SE), a combination of seizure types, and development of abnormal mental functioning. A 2-year seizure-free period is suggested for absence and rolandic epilepsy, whereas a 4-year seizure-free period is suggested for SP, CP, and absence seizures associated with tonic–clonic seizures. AED withdrawal generally is not suggested for patients with juvenile myoclonic epilepsy (JME), absence with clonic–tonic–clonic seizures, or clonic–tonic–clonic seizures. The AAN has issued guidelines for discontinuing AEDs in seizure-free patients.14 After assessing the risks and benefits to both the patient and society, AED withdrawal can be considered in a patient meeting the following profile: seizure free for 2 to 5 years, a history of a single type of partial seizure or primary GTC seizures, a normal neurologic exam and normal IQ, and an EEG that has normalized with treatment. When these factors are present, the relapse rate is expected to be less than 32% for children and 39% for adults.
Factors favoring successful withdrawal of AEDs include a seizure-free period of 2 to 4 years, complete seizure control within 1 year of onset, an onset of seizures after age 2 but before age 35, and a normal neurologic examination and EEG. Factors associated with a poor prognosis in discontinuing AEDs, despite a seizure-free interval, include a history of a high frequency of seizures, repeated episodes of status epilepticus (SE), a combination of seizure types, and development of abnormal mental functioning. A 2-year seizure-free period is suggested for absence and rolandic epilepsy, whereas a 4-year seizure-free period is suggested for SP, CP, and absence seizures associated with tonic–clonic seizures. AED withdrawal generally is not suggested for patients with juvenile myoclonic epilepsy (JME), absence with clonic–tonic–clonic seizures, or clonic–tonic–clonic seizures. The AAN has issued guidelines for discontinuing AEDs in seizure-free patients.14 After assessing the risks and benefits to both the patient and society, AED withdrawal can be considered in a patient meeting the following profile: seizure free for 2 to 5 years, a history of a single type of partial seizure or primary GTC seizures, a normal neurologic exam and normal IQ, and an EEG that has normalized with treatment. When these factors are present, the relapse rate is expected to be less than 32% for children and 39% for adults.
AED withdrawal should be done gradually, especially in patients with profound developmental disabilities. Some patients will have a recurrence of seizures as the AEDs are withdrawn. Sudden withdrawal is associated with the precipitation of SE. Withdrawal seizures are of particular concern for agents such as benzodiazepines and barbiturates. Seizure relapse has been reported to be more common if these AEDs are withdrawn over 1 to 3 months compared to over 6 months.
The risk of seizure relapse has been estimated at 10% to 70%. A meta-analysis determined that the relapse rate was 25% after 1 year and 29% after 2 years. Recurrence of seizures tends to occur early with at least one-half of the recurrences within 6 months of AED withdrawal and 60% to 90% within 1 year. Patients who relapse will generally become seizure free and in remission after AEDs are restarted although not necessarily immediately. The underlying epilepsy syndrome appears to determine prognosis for long-term remission.15
Clinical Controversy…
Nonpharmacologic Therapy
Nonpharmacologic therapy for epilepsy includes diet, surgery, and vagus nerve stimulation (VNS). A vagal nerve stimulator is an implanted medical device that is Food and Drug Administration (FDA) approved for use as adjunctive therapy in reducing the frequency of seizures in adults and adolescents older than 12 years of age with partial-onset seizures that are refractory to AEDs. It is also used off-label in the treatment of refractory primary generalized epilepsy. The mechanisms of antiseizure actions of VNS are unknown. Human clinical studies have shown that VNS changes the cerebrospinal fluid (CSF) concentration of inhibitory and stimulatory neurotransmitters and activates specific areas of the brain that generate or regulate cortical seizure activity through increased blood flow. There is experimental evidence to suggest that the anticonvulsant effect of VNS is mediated by the locus coeruleus.16
The VNS device is relatively safe. It may also have a positive effect on mood and behavior, often independent of seizure reduction. The most common side effect associated with stimulation is hoarseness, voice alteration, increased cough, pharyngitis, dyspnea, dyspepsia, and nausea. Serious adverse effects reported include infection, nerve paralysis, hypoesthesia, facial paresis, left vocal cord paralysis, left facial paralysis, left recurrent laryngeal nerve injury, urinary retention, and low-grade fever. In the VNS studies, the percentage of patients who achieved a 50% or greater reduction in their seizure frequency (responders) ranged from 23% to 50%.
![]() Surgery is the treatment of choice in selected patients with refractory focal epilepsy, especially those patients with seizures originating from the temporal lobe.17 The Early Randomized Surgery for Epilepsy trial resulted in freedom from seizures in 78% of newly refractory temporal lobe epilepsy patients, and none were seizure free in the group on standard drug therapy. Surgery reduces the risk of epilepsy-associated death, and it may also improve depression and anxiety in refractory epilepsy patients.18,19 A systematic review/meta-analysis of published evidence of temporal lobe patients with pharmacoresistant epilepsy concluded that the combination of surgery with medical treatment is four times as likely as medical treatment alone to achieve freedom from seizures.20 A National Institutes of Health Consensus Conference identified three absolute requirements for surgery. They are (a) an absolute diagnosis of epilepsy, (b) failure on an adequate trial of drug therapy, and (c) definition of the electroclinical syndrome. A focus in the temporal lobe has the best chance for a positive outcome; however, extratemporal foci can be excised successfully in more than 75% of patients. The procedure is not without risk. Learning and memory can be impaired postoperatively, and general intellectual abilities are also affected in a small number of patients. Surgery may be particularly useful in children with intractable epilepsy. Patients may need to continue AED therapy for a period of time following successful epilepsy surgery, but dosage reduction may be achieveable.21
Surgery is the treatment of choice in selected patients with refractory focal epilepsy, especially those patients with seizures originating from the temporal lobe.17 The Early Randomized Surgery for Epilepsy trial resulted in freedom from seizures in 78% of newly refractory temporal lobe epilepsy patients, and none were seizure free in the group on standard drug therapy. Surgery reduces the risk of epilepsy-associated death, and it may also improve depression and anxiety in refractory epilepsy patients.18,19 A systematic review/meta-analysis of published evidence of temporal lobe patients with pharmacoresistant epilepsy concluded that the combination of surgery with medical treatment is four times as likely as medical treatment alone to achieve freedom from seizures.20 A National Institutes of Health Consensus Conference identified three absolute requirements for surgery. They are (a) an absolute diagnosis of epilepsy, (b) failure on an adequate trial of drug therapy, and (c) definition of the electroclinical syndrome. A focus in the temporal lobe has the best chance for a positive outcome; however, extratemporal foci can be excised successfully in more than 75% of patients. The procedure is not without risk. Learning and memory can be impaired postoperatively, and general intellectual abilities are also affected in a small number of patients. Surgery may be particularly useful in children with intractable epilepsy. Patients may need to continue AED therapy for a period of time following successful epilepsy surgery, but dosage reduction may be achieveable.21
The ketogenic diet, devised in the 1920s, is high in fat and low in carbohydrates and protein, and it leads to acidosis and ketosis. Protein and calorie intake are set at levels that will meet requirements for growth. Most of the calories are provided in the form of heavy cream and butter. No sugar is allowed. Vitamins and minerals are supplemented. Medium-chain triglycerides can be substituted for the dietary fats. Fluids are also controlled. It requires strict control and parent compliance. Although some centers find the diet useful for refractory patients, others have found that it is poorly tolerated by patients. Long-term effects include kidney stones, increased bone fractures, and adverse effects on growth.22 An international consensus statement has been published, which offers recommendations employing various forms of the ketogenic diet which may be more tolerable, including the use of the modified Atkins diet and the Low Glycemic Index Treatment.23 Subsequent data support the use of these variations in the ketogenic diet, as well as the medium chain triglyceride ketogenic diet in select patients.24
Pharmacologic Therapy
Optimal management of epilepsy requires that AED treatment be individualized. Different patient groups (e.g., children, women of child-bearing potential, and the elderly) may be better suited to receive one AED than another by virtue not only of seizure type but also of susceptibility or relative risk for certain adverse effects. These issues are highlighted further below.
![]() Selection and optimization of AED therapy require not only an understanding of drug mechanism(s) of action and spectrum of clinical activity, but also an appreciation of pharmacokinetic variability and patterns of drug-related adverse effects. An AED must be effective for the specific seizure type being treated. The drug treatments of first choice depend on the type of epilepsy, drug-specific adverse effects, and patient preferences. Ultimately, AED effectiveness is the result of the interaction of each of these factors. A suggested algorithm for a general approach to the treatment of epilepsy is shown in Figure 40-1.
Selection and optimization of AED therapy require not only an understanding of drug mechanism(s) of action and spectrum of clinical activity, but also an appreciation of pharmacokinetic variability and patterns of drug-related adverse effects. An AED must be effective for the specific seizure type being treated. The drug treatments of first choice depend on the type of epilepsy, drug-specific adverse effects, and patient preferences. Ultimately, AED effectiveness is the result of the interaction of each of these factors. A suggested algorithm for a general approach to the treatment of epilepsy is shown in Figure 40-1.
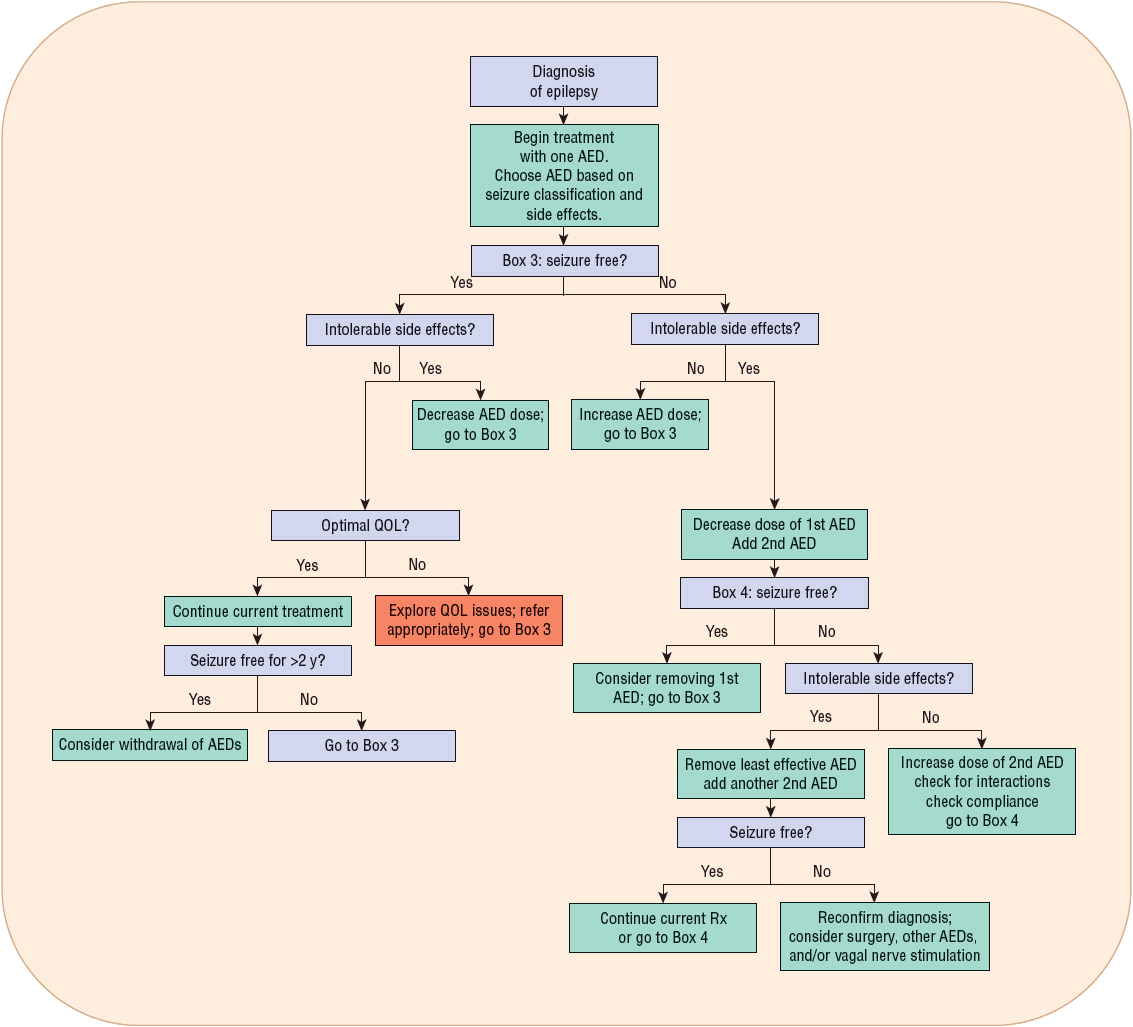
FIGURE 40-1 Algorithm for the treatment of epilepsy. (AED, antiepileptic drug; QOL, quality of life.)
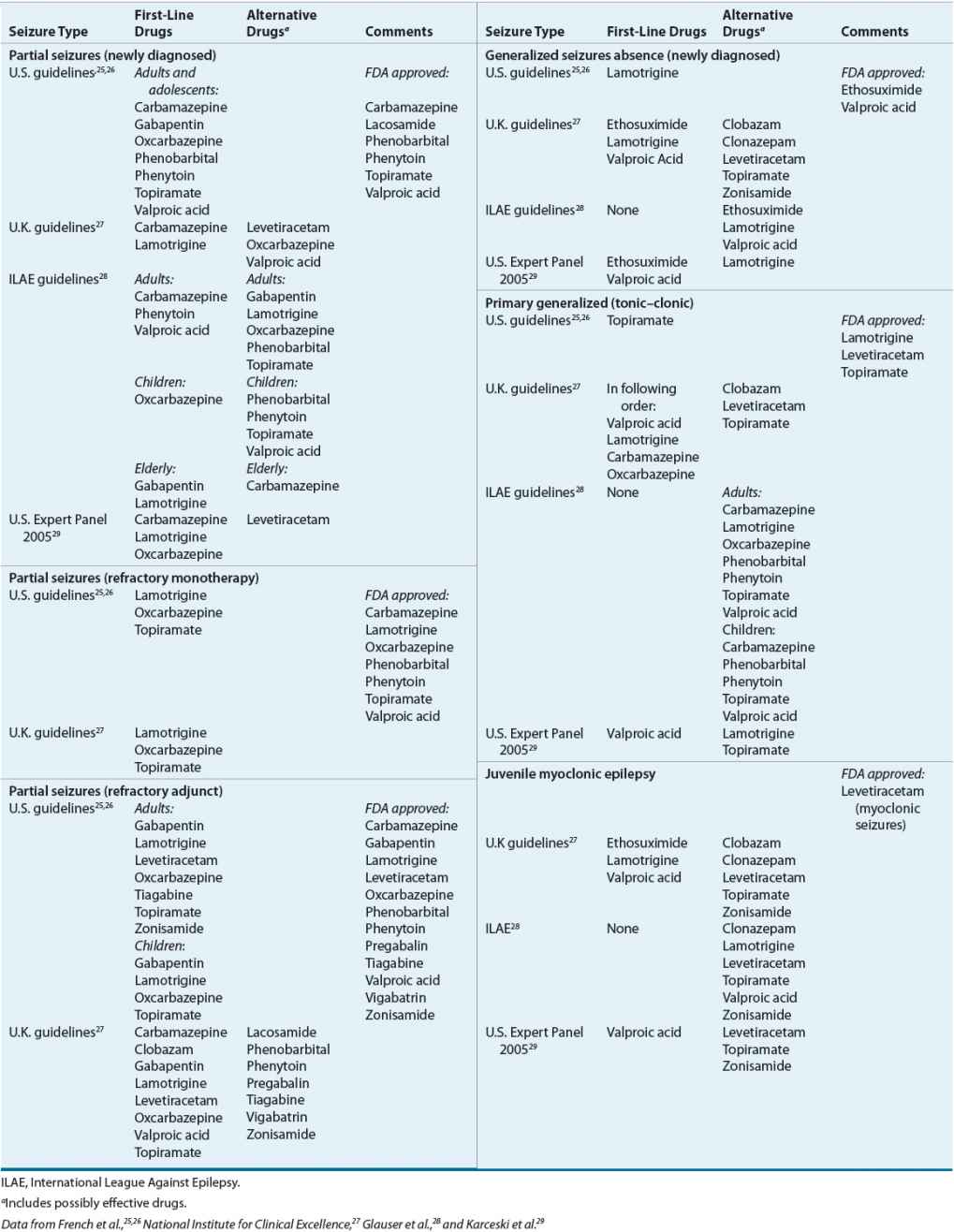
Table 40-3 provides evidenced-based treatment recommendations by three professional/regulatory bodies.25–28 In addition, recommendations from a U.S. panel of experts, which included more recent drug treatment data compared to the AAN–American Epilepsy Society (AES) recommendations are included.29
TABLE 40-3 Drugs of Choice for Specific Seizure Disorders
The mechanism of action of most AEDs can be categorized as (a) affecting ion channel kinetics, (b) augmenting inhibitory neurotransmission, or (c) modulating excitatory neurotransmission. Augmentation in inhibitory neurotransmission includes increasing CNS concentrations of GABA, whereas efforts to decrease excitatory neurotransmission are primarily focused on decreasing (or antagonizing) glutamate and aspartate neurotransmission. AEDs that are effective against GTC and partial seizures probably reduce sustained repetitive firing of action potentials by delaying recovery of sodium channels from activation. Drugs that reduce corticothalamic T-type calcium currents are effective against generalized absence seizures. Myoclonic seizures respond to drugs that enhance GABAA-receptor inhibition. In addition to mechanism of action, awareness of pharmacokinetic properties (Table 40-4), adverse effects (Table 40-5), and AED metabolic pathway as well as inducer or inhibitory effects on liver (Table 40-6) can aid in the optimization of AED therapy. Pharmacokinetic interactions are a common complicating factor in AED selection. Interactions can occur in any of the pharmacokinetic processes: absorption, distribution, metabolism, or elimination. Caution should be used when AEDs are added to or withdrawn from a drug regimen.
TABLE 40-4 Antiepileptic Drug Pharmacokinetic Data
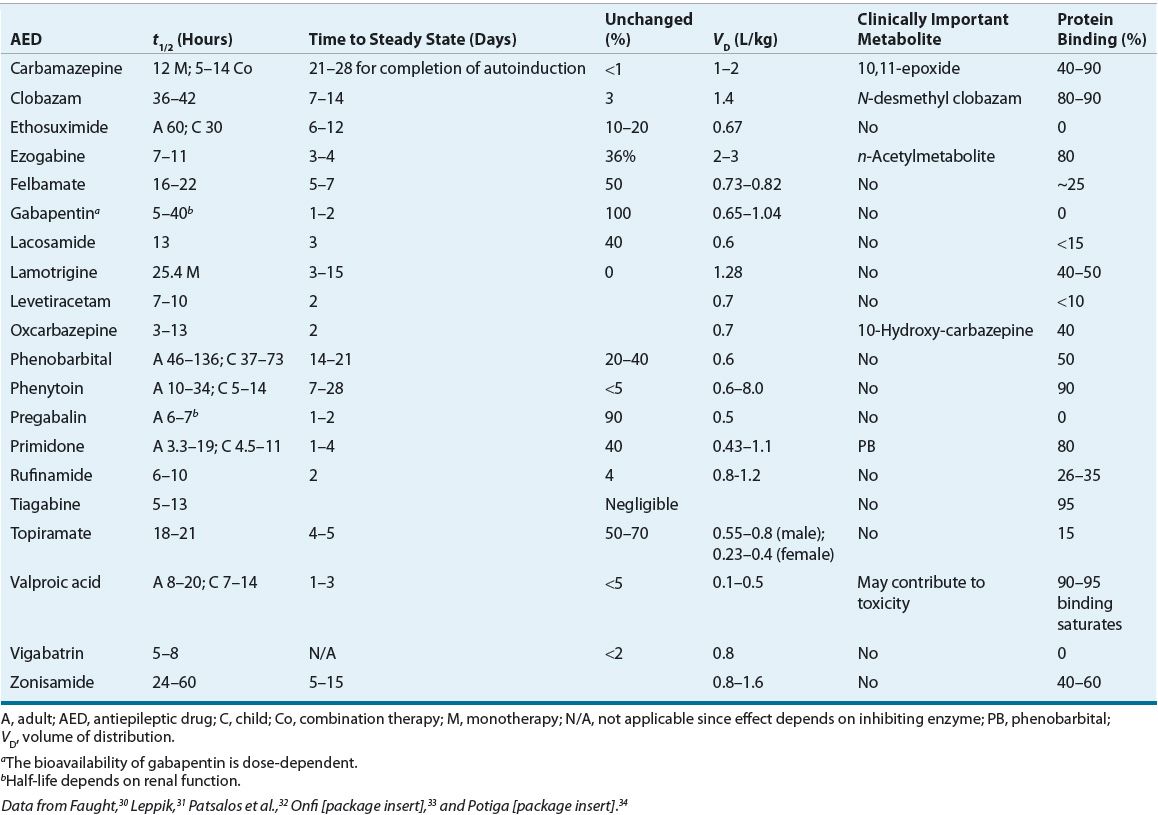
TABLE 40-5 Antiepileptic Drug Side Effects and Monitoring
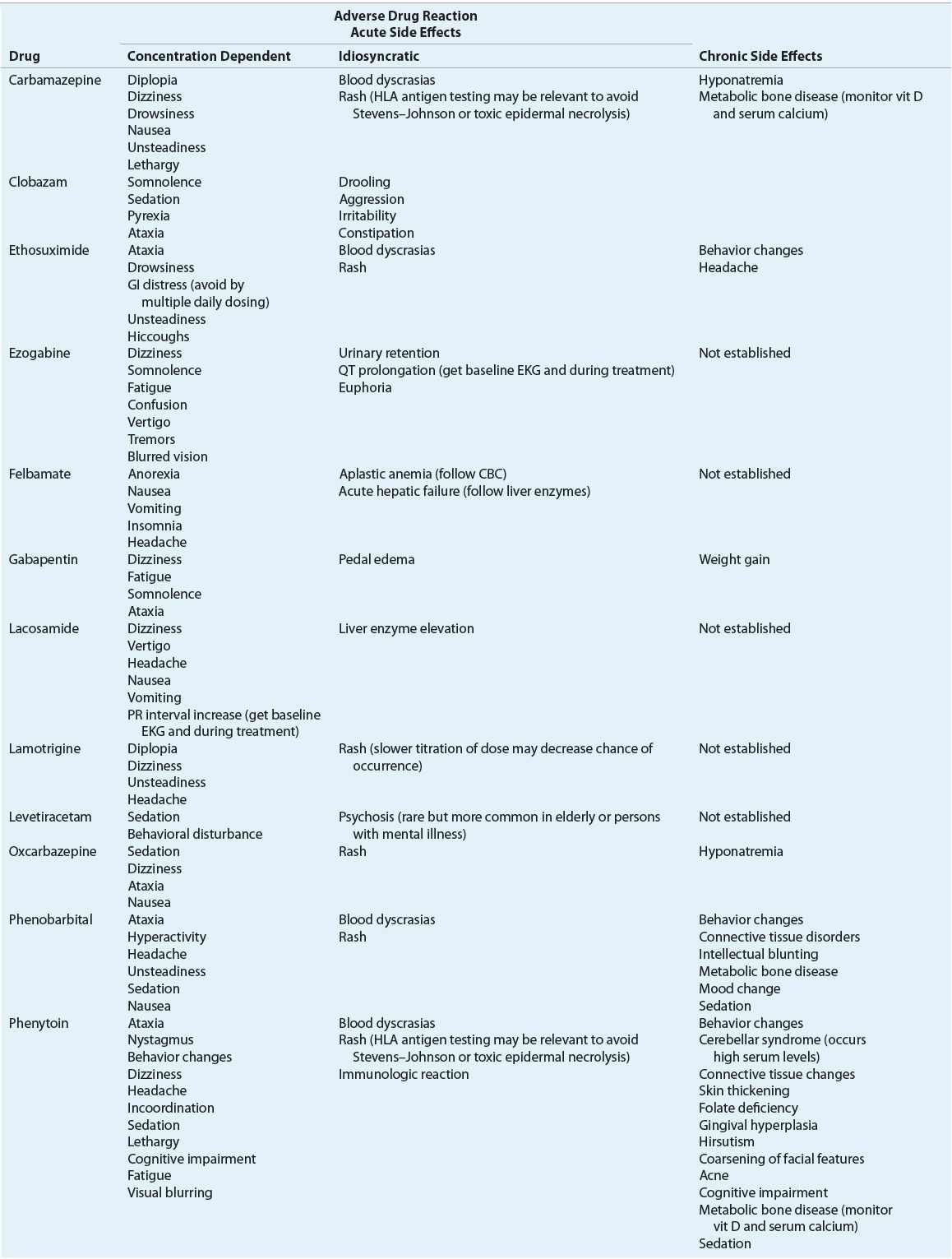
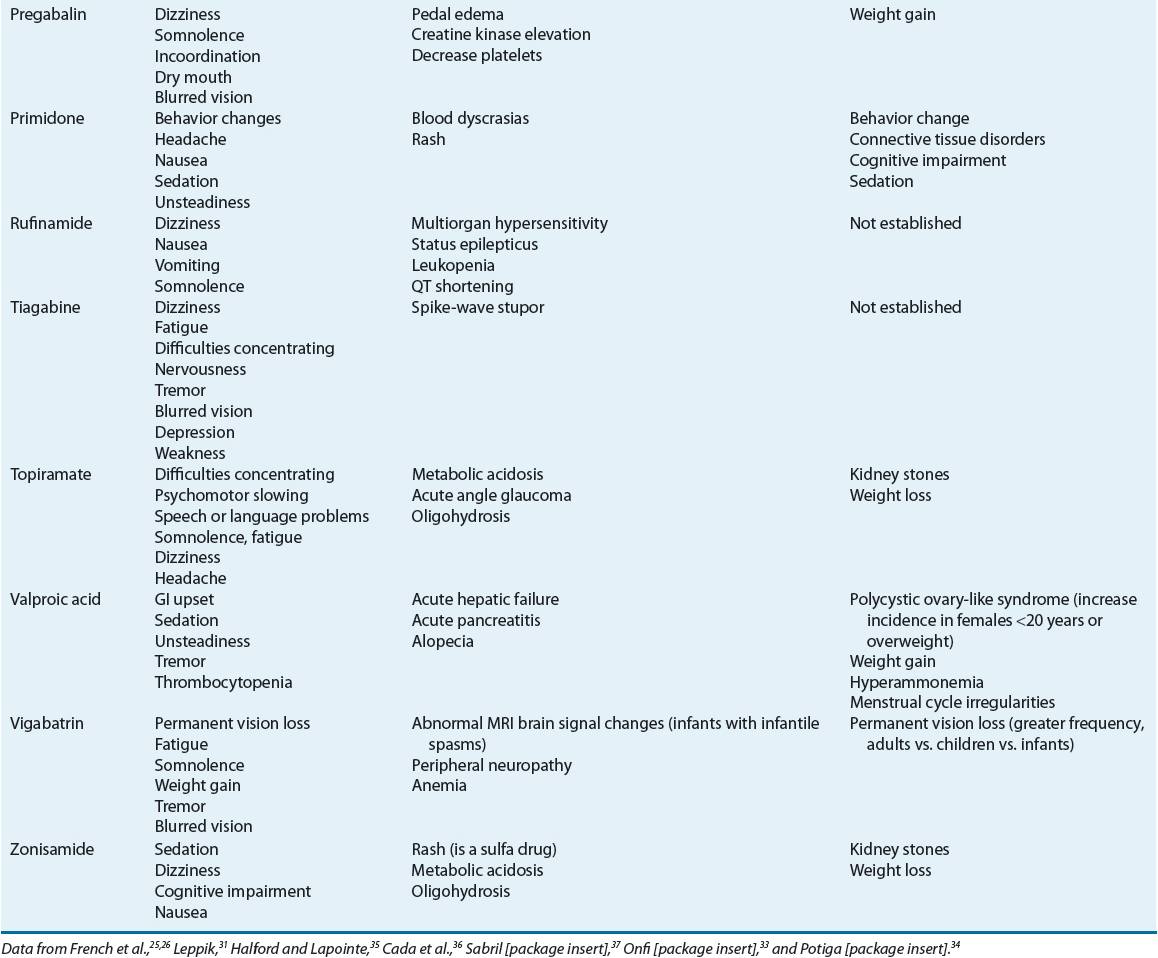
TABLE 40-6 Antiepileptic Drugs Elimination Pathways and Major Effects on Hepatic Enzymes
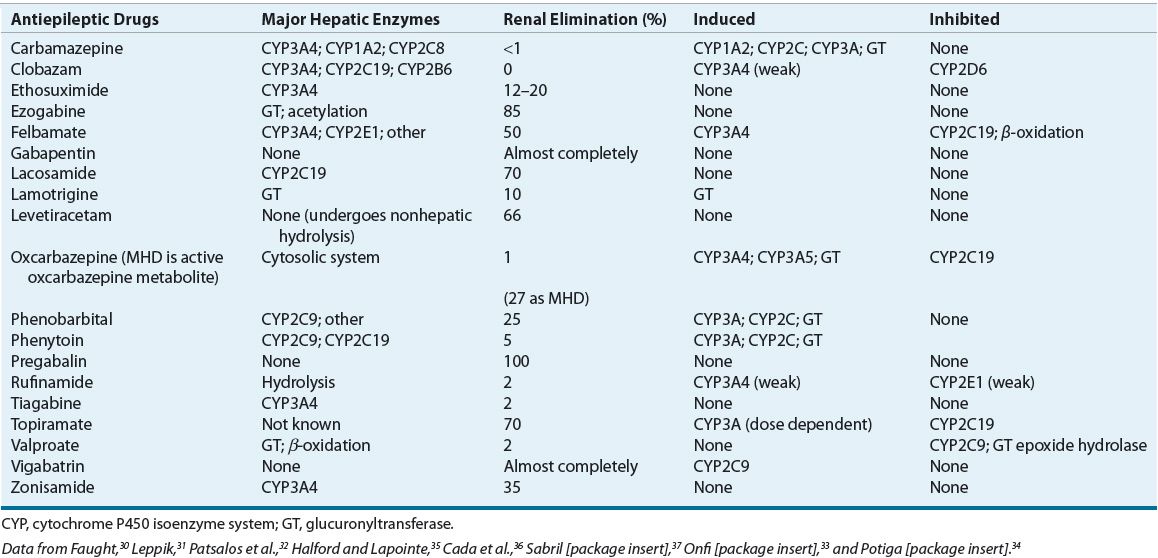
Adverse effects of AEDs can be divided into acute and chronic (see Table 40-5). Acute effects can be dose/serum concentration-related or idiosyncratic. Concentration-dependent effects are common and troublesome but not usually life-threatening. Neurotoxic adverse effects are encountered commonly and can include sedation, dizziness, blurred or double vision, difficulty with concentration, and ataxia. In many cases, these effects can be alleviated by decreasing the dose or avoided in some cases by titrating the dose upward very slowly. Most idiosyncratic reactions are mild, but they can be more serious if the hypersensitivity involves one or more organ systems. Other idiosyncratic side effects including hepatitis or blood dyscrasias are serious but rare.
Acute organ failure, when it occurs, generally occurs within the first 6 months of AED therapy. Unfortunately, laboratory screening evaluations of blood and urine typically are not helpful in predicting or detecting the early stage of severe reactions and generally are not recommended in asymptomatic patients. An exception to this is in the screening of patients of Southeast Asian heritage for HLA-B*1502 antigen who are to receive carbamazepine and possibly phenytoin, lamotrigine, and oxcarbazepine. There is a strong association between the presence of this antigen and Stevens–Johnson syndrome as well as toxic epidermal necrolysis.38 In addition the HLA genotype HLA-A*3101 has been found to be associated with multiple carbamazepine-induced cutaneous reactions in Chinese, Japanese, and European populations.38 In any patient, laboratory assessment, including white blood cell (WBC) counts and liver function tests, may be reasonable if the patient reports an unexplained illness (e.g., lethargy, vomiting, fever, or rash).7 It is important to note that patients dosed and maintained within “therapeutic ranges” are also capable of experiencing toxicities to AEDs.32 Another potential long-term adverse effect of AED treatment is osteomalacia and osteoporosis.39,40 The bone disorders associated with AED use are a heterogeneous group of disorders, ranging from asymptomatic high-turnover disease, with findings of normal bone mineral density, to markedly decreased bone mineral density sufficient to warrant the diagnosis of osteoporosis. While the etiology of these osteopathies is uncertain, it has been hypothesized that certain drugs, including phenytoin, phenobarbital, carbamazepine, oxcarbazepine, felbamate, and valproic acid, may interfere with vitamin D metabolism; at least for the CYP3A4 inducers this activity may be explained by recent findings of an inducible CYP3A4-dependent vitamin D pathway.41 Whether the other AEDs cause these effects is unknown, however, current evidence suggests that lamotrigine does not. Common laboratory findings in these patients include elevated bone-specific alkaline phosphatase concentration, intact parathyroid hormone, and decreased serum calcium and 25-OH vitamin D concentrations. Patients receiving these drugs should receive supplemental vitamin D and calcium, as well as bone mineral density testing if other risk factors for osteoporosis are present.
Comparative data now exist between some of the older AEDs, carbamazepine, phenytoin, and valproic acid and the newer agents, levetiracetam, lamotrigine, and topiramate (low dose) that suggest the older agents increase circulatory vascular risk markers, which may contribute to acceleration of atherosclerosis and that this effect is treatment duration dependent.42,43
The comparative effects of AEDs on cognition have been difficult to evaluate because of differences or inconsistencies in study design, seizure types studied, control for serum drug concentrations, and the neuropsychologic tests used. In general, there are not large differences between the older drugs, although the barbiturates, phenobarbital and primidone, appear to cause more cognitive impairment than other commonly used AEDs.44,45 Phenytoin, particularly when serum concentrations are above the commonly accepted therapeutic range, may have a greater effect on motor function and speed. Among the older AEDs, valproic acid may cause less impairment of cognition. Improvement in cognition has been reported in patients switched from phenytoin or phenobarbital to valproic acid. However, these improvements are subtle if patients are in the same relative area of the therapeutic range. Patients changed from polytherapy to monotherapy also may demonstrate improvement in cognition. Some of the newer agents are believed to cause fewer neurobehavioral or cognitive effects. Among the newer AEDs, gabapentin and lamotrigine have been shown in several studies to cause fewer cognitive impairments compared with older agents, such as carbamazepine.46–48 Conversely, topiramate may cause substantial cognitive impairment, particularly when used at high doses or during rapid dose escalation.48 In addition, these patients may not be fully aware of their deficits.49,50 AED treatment itself may sometimes worsen seizures due to improper AED selection for a specific seizure type or syndrome or can represent a paradoxical toxic effect of the drug.51
Because most adult patients have localization-related (partial-onset) seizures, the most widely used AEDs traditionally have been carbamazepine, phenobarbital, phenytoin, and valproic acid. For CP seizures, these AEDs have similar efficacy.52,53 Of these, carbamazepine and phenytoin are the most commonly prescribed AEDs for partial seizures in the United States. This preference is largely based on data derived from two landmark trials conducted through the Veterans Administration (VA) Epilepsy Cooperative Study Group. In the first of these trials, patients with new-onset partial or generalized epilepsy were randomized to receive either carbamazepine, phenobarbital, phenytoin, or primidone.52 After 3 years, patients who received either carbamazepine or phenytoin were equally likely, and patients on phenobarbital or primidone were least likely to have remained on their originally assigned treatment. Thus, carbamazepine and phenytoin were considered the drugs of first choice in patients with new-onset partial or generalized seizures. Carbamazepine was associated with fewer side effects. A followup study using almost identical methods compared carbamazepine and valproic acid.53 Carbamazepine- and valproic acid-treated groups had equal retention rates for tonic–clonic seizures. Carbamazepine was superior to valproic acid for efficacy in the treatment of partial seizures. Valproic acid caused slightly more adverse effects.
Based primarily on these trials, carbamazepine has been recognized as the AED of first choice for partial seizures. Several of the newer generation AEDs are now proving to be reasonable alternatives. The newer AEDs were first approved as adjunctive therapy for patients with refractory partial seizures. Monotherapy trials with several of these newer agents including lamotrigine, gabapentin, topiramate, oxcarbazepine, and levetiracetam have now been completed.54–56 Comparisons between lamotrigine and older agents, including carbamazepine and phenytoin as initial monotherapy for partial seizures have been conducted in Europe, and the results suggest comparable effectiveness and perhaps better tolerability for lamotrigine, particularly in elderly patients. In a large, unblinded, randomized, controlled trial in hospital-based outpatient clinics in the United Kingdom, lamotrigine was found to be clinically better than carbamazepine for time to treatment failure outcomes in newly diagnosed patients with partial seizures; lamotrigine was determined to be a cost-effective alternative to carbamazepine. Other drugs studied in this trial were gabapentin, oxcarbazepine, and topiramate.57 Results from a VA cooperative trial designed to compare gabapentin, lamotrigine, and carbamazepine in newly diagnosed elderly patients with partial seizures found that lamotrigine efficacy is comparable with that of both gabapentin and carbamazepine, and is better tolerated than carbamazepine but equal to gabapentin in this population.58 Clinical data suggest that in newly diagnosed patients, oxcarbazepine is as effective as phenytoin, valproic acid, and immediate-release carbamazepine, with perhaps fewer adverse effects. Close examination of the conversion to monotherapy trials suggests that oxcarbazepine demonstrates efficacy even in patients who previously had an inadequate response to carbamazepine, in spite of their structural similarity. Lastly, levetiracetam in a 1-year study was found to have equal efficacy and tolerability when studied against controlled-release carbamazepine.59
In addition, several monotherapy trials using an active control or pseudoplacebo design also have been conducted. Although these study designs provide evidence of efficacy for the newer drugs, because the comparison is between active drug and placebo in patients who continue to have seizures in spite of current treatment with standard AEDs, it is difficult to compare the efficacy of the newer drugs directly with the older AEDs. Generally speaking, the newer AEDs appear to have comparable efficacy to the older agents and are perhaps better tolerated. A recent systematic review and meta-analysis attempted to compare the efficacy and tolerability of the newer AEDs in treatment of refractory partial epilepsy as add on therapy with another AED or placebo; although the results provided indirect evidence, the authors found topiramate and probably levetiracetam more efficacious in controlling seizure frequency and gabapentin less efficacious compared to all other new AEDs. In addition, tolerability was poorer with oxcarbazepine and topiramate whereas gabapentin and levetiracetam were better tolerated with the most common side effects comparable between the new AEDs.60
To date, among the newer generation agents, lamotrigine, oxcarbazepine, and topiramate have received FDA approval for use as monotherapy in patients with partial seizures. Phenobarbital and primidone are also useful in partial seizures, but sedation and cognitive adverse effects limit their utility. Felbamate, which has monotherapy approval, is effective but has been associated with some significant side effects. Interpretation of monotherapy trials with the newer AEDs can be daunting owing to the unique study designs and specific patient populations employed. Withholding an effective AED (i.e., giving placebo) in patients with epilepsy is generally considered unethical. Primarily generalized seizures such as absence seizures may respond differently pharmacologically than other seizure types. Phenytoin, phenobarbital, and carbamazepine, although effective in GTC and partial seizures, are ineffective for absence seizures, and in some cases, can precipitate an increase in seizure frequency. Absence seizures are best treated with ethosuximide, valproic acid, and perhaps lamotrigine. For levetiracetam, topiramate, and zonisamide, additional data are needed to confirm efficacy. Oxcarbazepine, gabapentin, and tiagabine do not appear to be effective in treating absence seizures, and can worsen this condition in some patients. If the patient has a combination of absence and other generalized or partial seizures, valproic acid or lamotrigine is the preferred first choice because they are effective for absence and other seizure types. If valproic acid is ineffective in treating a mixed seizure disorder that includes absence, ethosuximide could be used in combination with another AED.
The traditional treatment of tonic–clonic seizures is phenytoin; however, carbamazepine and valproic acid are increasingly used because these AEDs have a lower incidence of side effects with equal efficacy. Valproic acid generally is considered the drug of first choice for atonic seizures and for JME. Lamotrigine and perhaps topiramate and zonisamide can be alternative agents for these seizure types. Levetiracetam is FDA approved as adjunctive treatment of myoclonic seizures in patients with JME.
Results of a large, unblinded, randomized, controlled trial conducted in patients with new-onset generalized and unclassified epilepsy outpatients in the United Kingdom has helped to define the role of the newer generation drugs. These researchers found that for idiopathic generalized epilepsy, valproic acid was significantly better tolerated than topiramate and more efficacious than lamotrigine.61 A post hoc analysis of this trial delineated clinical factors significant in influencing treatment failure and in achieving a 12 month remission.62
Serum concentrations of the older AEDs should be viewed as a tool with which to optimize therapy for an individual patient, not as a therapeutic end point in itself. The serum concentration is a target that should be correlated with clinical response. The desired outcome is the cessation of seizures without side effects. Seizure control can occur before the “minimum” of the published therapeutic range is achieved, and side effects can appear before the “maximum” of the range is achieved. Some patients may need and tolerate concentrations beyond the maximum. The therapeutic range for AEDs can be different for different seizure types. Serum concentrations may need to be higher to control CP seizures than to control tonic–clonic seizures. Clinicians should define a therapeutic range for an individual patient above which there are side effects and below which the patient experiences seizures. Then serum levels can be useful to document lack of efficacy, loss of efficacy, noncompliance, and to determine how much room there is to increase a dose based on expected toxicity. Depending on the AED, serum levels can also be useful in patients with significant renal and/or hepatic disease, patients taking multiple drugs, and women who are pregnant or taking oral contraceptives (OCs). Therapeutic concentration ranges have not been clearly defined for some of the second-generation AEDs.
Therapeutic Considerations in the Elderly and Young
Use of AEDs in the elderly and young can pose special challenges.32 Avoidance of AEDs that interact with other medications that the elderly are often taking is of upmost importance. Many of the AEDs are inducers or inhibitors of the cytochrome P450 (CYP450) system, which can adversely affect the drug level of concomitantly administered drugs. Hypoalbuminemia is common in the elderly, and can make monitoring and adjustment of serum drug levels of highly albumin-bound AEDs, such as phenytoin, valproic acid, and tiagabine, problematic. The elderly also experience body mass changes, such as an increase in fat to lean body mass or decrease in body water, which can affect the volume of distribution of some drugs, and therefore possibly the elimination half-life. In addition, declining renal and/or hepatic function can occur in the elderly, which can require a lower dose of the AED. Lastly, the pharmacodynamic response to AEDs can change with aging such that elderly patients may be more sensitive to various neurocognitive adverse effects. Also, elderly patients’ seizures may be controlled at relatively lower total serum concentrations.
For neonates and infants, an increase in the total body water to fat ratio and a decrease in serum albumin and α-acid glycoprotein can result in volume of distribution changes that can affect the elimination half-life of the AEDs. In addition, newborns up to the age of 2 to 3 years display decreased efficiency in renal elimination, with newborns being the most affected. Hepatic activity is also reduced in this population. However, by age 2 to 3 years, hepatic activity is more robust than that seen in adults. Therefore, children require higher doses of many of the AEDs than adults, whereas neonates and infants require lower doses. Lastly, rapidly changing and sometimes inconsistent metabolism in the patient groups above make therapeutic drug monitoring especially important even though the definition of therapeutic blood level is less certain in these patients than in adults.
Therapeutic Considerations in Women (and Men)
Many hormones influence brain electrical excitability, and estrogen and progesterone may interact in complex ways to alter neuronal excitability and protein synthesis. Estrogen has a slight proconvulsant effect, whereas progesterone exerts a mild anticonvulsant effect. Estrogen has a mild inhibitory effect on GABA receptors, potentiates excitatory glutaminergic activity, and can promote the development of kindling. Progesterone has the opposite effect and appears to potentiate GABA receptor activity and reduce neuronal discharge rates. AEDs, especially hepatic metabolizing enzyme inducers, increase the metabolism of these hormones and induce the production of sex hormone-binding globulin. This may lead to decreases in the unbound fraction of the hormone. Enzyme-inducing AEDs, including topiramate and oxcarbazepine at higher doses, can cause treatment failures in women taking OCs owing to induction of the metabolism of ethinyl estradiol and progestin. This may also be an issue with rufinamide, lamotrigine, clobazam, and felbamate, all which have a small effect in decreasing the bioavailability of OCs. A supplemental form of birth control, in addition to OCs, is advised if breakthrough bleeding occurs. However medroxyprogesterone depot injection, copper intrauterine devices, and hormone-releasing intrauterine systems are not affected by AEDs. There are no data available on the efficacy of the transdermal contraceptive patch or the emergency contraceptive pill in patients taking these AEDs, but it has been suggested that women use twice the normal dose of the postcoital pill.63 Valproic acid, benzodiazepines, except clobazam, and most of the newer AEDs, such as gabapentin, levetiracetam, tiagabine, zonisamide, vigabatrin, and lacosamide, are not enzyme inducers and have not been implicated in reducing contraceptive effectiveness. Of note, OCs lower lamotrigine’s serum level significantly and lower valproic acid’s level about 20%.32
In some women, vulnerability to seizures is highest just before and during the menstrual flow (catamenial seizures) and at the time of ovulation. The increased susceptibility to seizures during those catamenial periods is associated with a slight increase of estrogen relative to progesterone. The risk of catamenial epilepsy is estimated at 12.5%, but it may be as high as 50% in women with epilepsy. This pattern of seizure exacerbation can also be related to progesterone withdrawal and changes in the estrogen-to-progesterone ratio. Conventional AEDs should be used as primary agents but intermittent supplementation with higher dose of AED or benzodiazepines should be considered. Acetazolamide also has been used during catamenial periods but with variable and limited success. Hormonal therapy with progestational agents, particularly cyclic natural progesterone therapy, may be effective.
Reproductive endocrine disorders are common in women with epilepsy and include menstrual irregularity, infertility, sexual dysfunction, and in some patients polycystic ovary syndrome (PCOS).64 Potential mechanisms for these disturbances include disruption of the hypothalamic–pituitary–adrenal (HPA) axis via seizure discharges in limbic structures and/or AEDs.64 AEDs, particularly the enzyme-inducing agents (e.g., carbamazepine, phenytoin, and phenobarbital), also may affect HPA function by altering the metabolism of the neuroactive sex hormones, including testosterone. Valproic acid is associated with increasing changes in sex hormone concentrations that causes hyperandrogenism and polycystic changes regardless if the patient has epilepsy, especially in women who have gained weight or those who start valproic acid at age less than 20 years.64
During pregnancy there may be increased maternal seizures, pregnancy complications, and adverse fetal outcome.65 Approximately 25% to 30% of pregnant women have increased seizures, whereas seizures decrease in a similar number. However, the risk of seizures is significantly less if the patient has been seizure free 12 months prior to the pregnancy.66 Increased seizure frequency may result from either a direct effect on seizure threshold or a reduction in AED concentration. An increase in clearance has been reported for phenytoin, carbamazepine, phenobarbital, ethosuximide, lamotrigine, oxcarbazepine, levetiracetam, topiramate, and clorazepate during pregnancy. Protein binding may also be reduced. The altered disposition of AEDs can begin as early as the first 10 weeks of pregnancy, and may require up to 4 weeks postpartum to normalize (longer for carbamazepine and phenobarbital than for phenytoin).
Women with epilepsy have a higher incidence of adverse pregnancy outcomes. Although the risk of congenital malformations is 4% to 6% (twice as high as in nonepileptic women), more than 90% of pregnancies in epileptic mothers have satisfactory outcomes. Older data, much of which included AED polytherapy, indicated that barbiturates and phenytoin may cause congenital heart malformations, orofacial clefts, and other malformations. From these data the risk of neural tube defect with valproic acid and carbamazepine was estimated to be 0.5% to 1%, respectively, and appeared to be related to drug exposure during gestational days 0 to 28. Other adverse pregnancy outcomes associated with maternal seizures, but not necessarily caused by AEDs, are growth, psychomotor, and mental retardation. Women with epilepsy are also more likely to have miscarriages, and 10% to 20% of infants are born with low birth weight. Updated practice parameters are available to aid in the counseling and management of pregnant women with epilepsy.67–69
Although data exist which question the effectiveness of folic acid supplementation, it is currently believed that some teratogenic effects may be prevented by adequate folate intake; therefore, prenatal vitamins with folic acid (0.4 to 5 mg/day) should be given to any woman of child-bearing potential who is taking AEDs.65 Also, that higher folate doses should be used in women with a history of a previous pregnancy with a neural tube defect or taking valproic acid. Higher AED doses and serum concentrations, polytherapy, and a family history of birth defects appear to increase the teratogenic risk of AEDs. Deciding on the most effective single-drug treatment prior to conception is vitally important. Current data strongly suggest that an increased risk of adverse outcomes in women with epilepsy is due to teratogenic effects of AEDs and not epilepsy since studies show that epileptic women who do not take AEDs have the same risk of birth defects as infants born to control, seizure free women.70 The most concerning effects are found with the use of valproic acid in the pregnant patient. Data gathered from pregnancy registries and long-term studies suggest that valproic acid exposure is associated with a 1% to 2% risk of neural tube defects, a 10- to 20-fold increase over the general population, and an increased risk of neurodevelopmental deficits, reduced verbal abilities, and poorer attentional tasks; it appears that these effects are dose-dependent with major congenital malformation risk significantly increasing at 600 mg/day and largest risk observed at doses that exceed 1,000 mg/day. However, individual susceptibility is genetically determined, and teratogenicity can occur at much lower doses in some persons. Data are still limited on the newer agents, although topiramate may have a negative effect on birth weight and cause an increase risk in oral cleft and hypospadias in the fetus.70 Some AEDs can cause neonatal hemorrhagic disorder, which can be prevented by administrating 10 mg/day vitamin K orally to the mother during the last month of pregnancy and/or administering parenteral vitamin K to the newborn at delivery.69
Most AEDs pass into the breast milk, and concentrations are measureable in breastfeeding infants. In general, the degree of protein binding of a given AED allows for prediction of its concentration in breast milk. AEDs with less protein binding accumulate more in breast milk. Treatment with AEDs is not necessarily a reason to discourage breastfeeding. In fact, an argument could be made that since AEDs should rarely be discontinued abruptly, breastfeeding is a reasonable way to allow for a downward titration of a medication that the baby was exposed to for the past 9 months. Infants born to women taking any AED (particularly barbiturates or benzodiazepines) should be closely observed for signs of excess sedation, irritability, or poor feeding.65 An ongoing multicenter observational study of breastfeeding women taking AED monotherapy has failed to show significant cognitive effects in children exposed in utero to various AEDs—carbamazepine, lamotrigine, phenytoin, or valproic acid, although there was some negative effect on cognition noted for phenytoin.71
The perimenopausal period can be associated with worsening of seizures. At menopause, seizures often improve in frequency, particularly in women with a catamenial seizure pattern. According to current data, conjugated equine estrogens plus 2.5 mg of medroxyprogesterone acetate may increase the frequency of epileptic seizures. It is suggested that a combination of single estrogenic compound such as 17-β-estradiol along with a natural progesterone should be considered in women who need hormone replacement therapy for disruptive menopausal symptoms.72 Data suggest that men with epilepsy have reduced fertility, and that carbamazepine, oxcarbazepine, and valproic acid are associated with sperm abnormalities in these men while levetiracetam appears to slightly increase serum testosterone.73 In addition, valproic acid seems to cause testicular atrophy resulting in reduced testosterone volume.74
Clinical Considerations with Specific Drugs
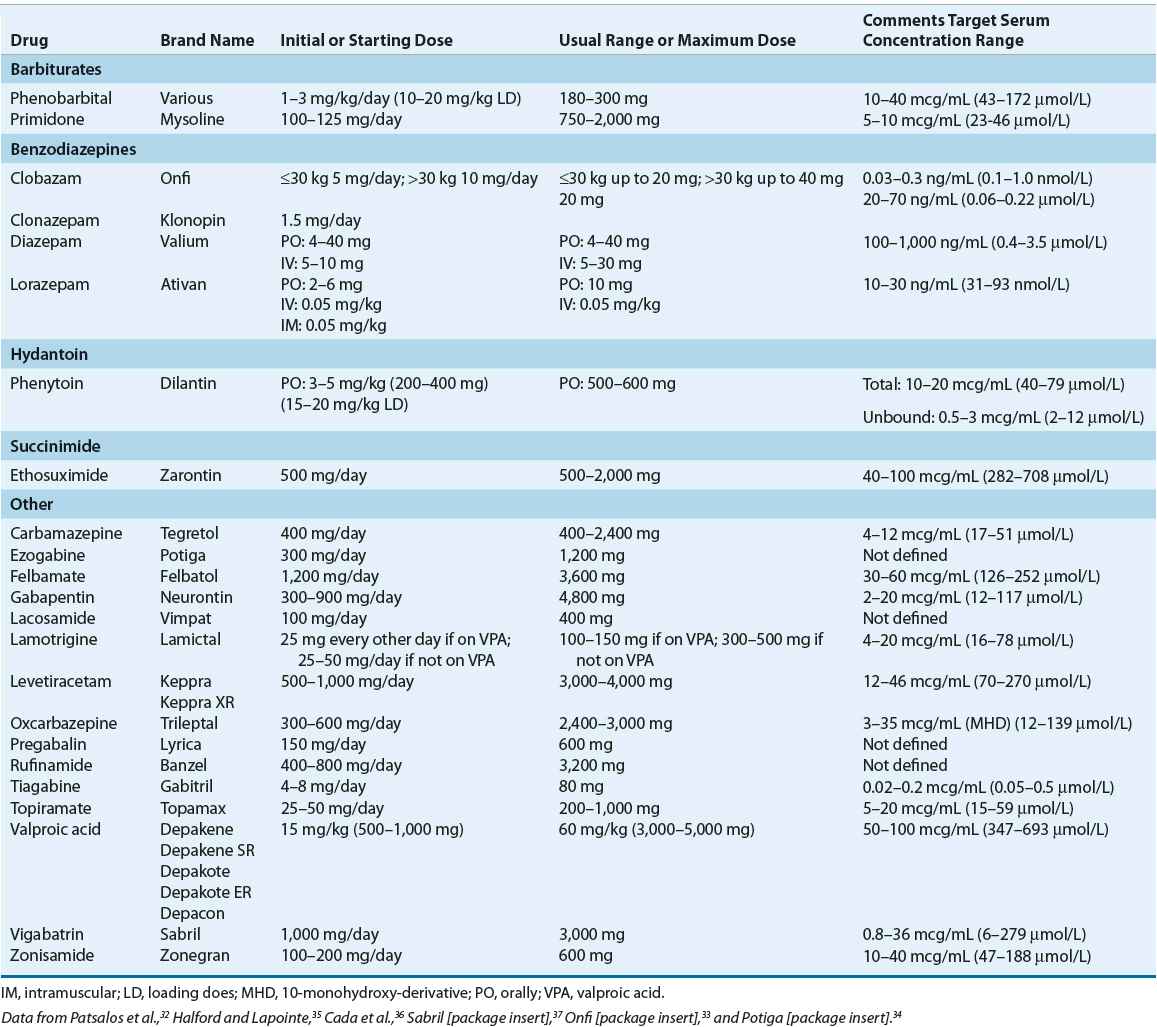
Tables 40-4 through 40-7 list specific data (including pharmacokinetics, adverse effects, metabolism, and dosing) for each of the commonly used AEDs. Below we summarize the pharmacology, advantages and disadvantages, and perspectives on the place in therapy of some specific AED.
TABLE 40-7 Antiepileptic Drugs Dosing and Target Serum Concentration Ranges