Enucleation of Pancreatic Neuroendocrine Tumor
Megan B. Anderson
Christopher D. Raeburn
Barish H. Edil
DEFINITION
Pancreatic neuroendocrine tumors (PNETs) are a diverse group of neoplasms that can arise from mature endocrine cells of the pancreas (α, β, δ, and γ cells) and multipotent cells, which have the ability to differentiate into endocrine and exocrine cells. PNETs were previously known as islet cell tumors.
PNETs can be subdivided into functional and nonfuctional based on their production of specific pancreatic endocrine hormones. Functional PNETs can produce insulin, gastrin, glucagon, vasoactive intestinal polypeptide (VIP), and somatostatin.
DIFFERENTIAL DIAGNOSIS
Functional PNETs amenable for enucleation are the following:
Insulinoma
Selected gastrinoma
Selected somatostatinoma
Selected nonfunctional PNETs amenable for enucleation are typically less than 2 cm but this is an area of controversy.
PATIENT HISTORY AND PHYSICAL FINDINGS
A thorough history and physical must be obtained to assess for signs of functional tumors, symptoms of mass effect, or evidence of metastatic disease. Physical exam should focus on abdominal masses, organomegaly, signs of biliary obstruction or liver dysfunction, and lymphadenopathy.
Although most PNETs are sporadic, 10% can be associated with predisposing syndromes such as multiple endocrine neoplasia type 1 (MEN-1), von Hippel-Lindau (VHL) disease, neurofibromatosis type 1 (NF1), and tuberous sclerosis complex (TSC).
Nonfunctional PNETs are typically slow growing and occur in the head of the pancreas. These lesions usually present similarly to pancreatic adenocarcinoma due to the mass effect of the tumor: jaundice, abdominal pain, and weight loss.
Functional PNETs
Insulinoma: Whipple’s triad—symptoms of hypoglycemia, generally when fasting, at night, or during exercise; hypoglycemia documented at the time of symptoms; and symptom relief with glucose administration.
Supervised 72-hour fast with a plasma glucose of less than 45 mg/dL
Increased C peptide more than 100 pmol/L
Negative sulfonylurea screen in urine
Negative insulin antibodies
Gastrinoma: Zollinger-Ellison syndrome—refractory peptic ulcer disease and secretory diarrhea Serum gastrin of more than 500 pg/mL
Gastric pH less than 3
Positive secretin or calcium stimulation test
IMAGING AND OTHER DIAGNOSTIC STUDIES
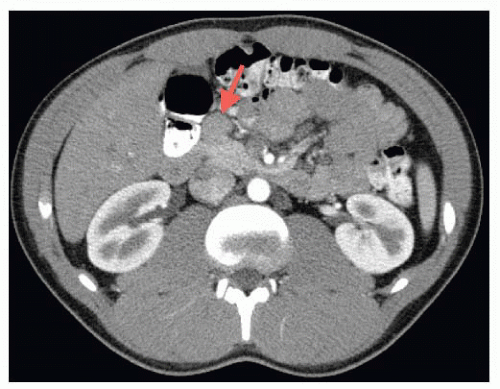
Imaging: Computerized tomography (CT), magnetic resonance imaging (MRI), endoscopic ultrasonograph with fine needle aspiration (EUS/FNA), somatostatin receptor scintigraphy (SRS), percutaneous transhepatic portal venous sampling (PTPVS), and arterial stimulation with venous sampling (ASVS) all may play a role in localization of a PNET and preoperative planning. FIG 1 shows a CT scan of an insulinoma at the level of the head of the pancreas. These lesions are typically best visualized on arterial phase CT.
CT scanning is unfortunately only 67% sensitive in localizing PNET and these other adjunctive measures may be required.
As a part of EUS evaluation, tattoo small lesions or lesions that are deep within the parenchyma of the pancreas to make localization easier at the time of operation.
Based on the data collected from laboratory tests and imaging studies, only select PNETs are amenable to enucleation. Low-grade tumors such as insulinomas are most amenable to enucleation.
SURGICAL MANAGEMENT
Preoperative Planning
Because most (>90%) insulinomas are benign, enucleation is usually preferred. A formal pancreatic resection should be considered if there is evidence of invasion, lymphadenopathy, or if the tumor is in close proximity to the pancreatic
duct or major vessels. It is possible to perform an enucleation through traditional open technique or laparoscopically.
For lesions where the pancreatic duct may be at risk for injury, some have advocated preoperative placement of a pancreatic duct stent. There is not clinical data that supports or condemns this approach.
Contraindications would include recent pancreatitis, uncontrolled coagulopathy, comorbidities that would limit life expectancy, and signs of malignancy, which include enlarged lymph nodes or distant metastases.
Positioning
The patient should be placed supine on the operating room table. The entire abdomen should be sterilely prepped and draped.
TECHNIQUES
PLACEMENT OF INCISION
Open: An upper midline incision should be made to enter the abdomen.
Laparoscopic: An infraumbilical 10-mm port should be placed to gain access to the abdomen. From there, another two 10-mm ports will be placed in the left upper quadrant and right lower quadrant, and two 5-mm ports will be placed in the right upper quadrant and left lower quadrant as shown in FIG 2.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree