Enteral Nutrition
KEY CONCEPTS
![]() The GI tract defends the host from toxins and antigens by both immunologic and nonimmunologic mechanisms, collectively referred to as the gut barrier function. Whenever possible, enteral nutrition (EN) is preferred over parenteral nutrition (PN) because it is as effective, may reduce metabolic and infectious complications, and is less expensive.
The GI tract defends the host from toxins and antigens by both immunologic and nonimmunologic mechanisms, collectively referred to as the gut barrier function. Whenever possible, enteral nutrition (EN) is preferred over parenteral nutrition (PN) because it is as effective, may reduce metabolic and infectious complications, and is less expensive.
![]() Candidates for EN are those with a sufficiently functioning GI tract to allow adequate nutrient absorption who cannot or will not eat and in whom enteral access can be safely obtained.
Candidates for EN are those with a sufficiently functioning GI tract to allow adequate nutrient absorption who cannot or will not eat and in whom enteral access can be safely obtained.
![]() The most common route for both short- and long-term EN access is directly into the stomach. The method of delivery may be either continuously via an infusion pump, intermittently via a pump or gravity drip, or by gravity or syringe bolus administration.
The most common route for both short- and long-term EN access is directly into the stomach. The method of delivery may be either continuously via an infusion pump, intermittently via a pump or gravity drip, or by gravity or syringe bolus administration.
![]() Patients unable to tolerate feeding directly into the stomach because of impaired gastric motility and for those at high risk of aspiration, feeding tube tip placement into the duodenum or jejunum may be indicated. When feeding into the small bowel, the continuous method of delivery via an infusion pump is required to enhance tolerance.
Patients unable to tolerate feeding directly into the stomach because of impaired gastric motility and for those at high risk of aspiration, feeding tube tip placement into the duodenum or jejunum may be indicated. When feeding into the small bowel, the continuous method of delivery via an infusion pump is required to enhance tolerance.
![]() Selection of the enteral feeding formulation depends on nutritional requirements, the patient’s primary disease state and related complications, and nutrient digestibility and absorption. A standard polymeric formulation will meet the needs of the majority of adults and children.
Selection of the enteral feeding formulation depends on nutritional requirements, the patient’s primary disease state and related complications, and nutrient digestibility and absorption. A standard polymeric formulation will meet the needs of the majority of adults and children.
![]() Measurement of gastric residual volumes can be used to monitor GI tolerance in patients receiving gastric feeding. Although not always reliable, excessive residual volumes may be associated with nausea, abdominal distension, and increased aspiration risk.
Measurement of gastric residual volumes can be used to monitor GI tolerance in patients receiving gastric feeding. Although not always reliable, excessive residual volumes may be associated with nausea, abdominal distension, and increased aspiration risk.
![]() Management of diarrhea in patients receiving EN should focus on identification and correction of the most likely cause(s). Tube feeding-related causes include too rapid delivery or advancement, intolerance to the formula composition, and occasionally formula contamination.
Management of diarrhea in patients receiving EN should focus on identification and correction of the most likely cause(s). Tube feeding-related causes include too rapid delivery or advancement, intolerance to the formula composition, and occasionally formula contamination.
![]() Prior to administering medications through a feeding tube, the feeding tube tip location should be verified (stomach or small bowel) and the most suitable dosage form selected. Medications that should not be crushed and administered through a tube include enteric-coated or sustained-release capsules or tablets and sublingual or buccal tablets.
Prior to administering medications through a feeding tube, the feeding tube tip location should be verified (stomach or small bowel) and the most suitable dosage form selected. Medications that should not be crushed and administered through a tube include enteric-coated or sustained-release capsules or tablets and sublingual or buccal tablets.
![]() The coadministration of medications with EN can result in alterations in bioavailability and/or changes in the desired pharmacologic effects. Medications known to interact with EN include phenytoin, warfarin, selected antibiotics, antacids, and proton-pump inhibitors.
The coadministration of medications with EN can result in alterations in bioavailability and/or changes in the desired pharmacologic effects. Medications known to interact with EN include phenytoin, warfarin, selected antibiotics, antacids, and proton-pump inhibitors.
INTRODUCTION
Enteral nutrition (EN) is defined as the delivery of nutrients by tube or by mouth into the GI tract. This chapter focuses on nutrient delivery through a feeding tube rather than the oral ingestion of food. The terms enteral nutrition and tube feeding are thus used interchangeably in this context. The goal of EN is to provide calories, macronutrients, and micronutrients to those patients who are unable to achieve these requirements from an oral diet. Improvements in enteral access techniques and feeding formulations over the past 20 to 30 years and the recognition of methods to prevent and manage complications have resulted in an increased use of EN across all healthcare settings.
In this chapter, principles and practices related to the successful use of EN support are described. Digestive and absorptive physiology is reviewed, and the beneficial effects of EN are presented. The indications for EN and descriptions of various enteral access and administration methods are also summarized. Characteristics of commercially available enteral feeding formulations are presented, as well as administration and monitoring guidelines. Strategies to prevent and manage complications are discussed, and clinical therapeutic controversies are highlighted. In addition, issues of drug compatibility, drug–nutrient interactions, and drug administration via feeding tubes are discussed. Finally, the effectiveness and pharmacoeconomics of EN in enhancing nutrition and disease outcome goals are reviewed.
GASTROINTESTINAL TRACT PHYSIOLOGY
The GI tract plays a key role in the processing of ingested foods many of which are modifiable by the presence of acute and chronic illnesses.
Digestion and Absorption
Digestion and absorption are GI processes that generate the body’s usable fuels.1,2 Digestion consists of the stepwise conversion of a complex chemical and physical nutrient into a molecular form that is absorbable by the intestinal mucosa. Absorption from the GI tract is a multistep process that includes the transfer of a nutrient across the intestinal cell membrane. The nutrient ultimately reaches the systemic circulation through the portal venous or splanchnic lymphatic systems, provided that the GI or biliary tract does not excrete it. Ingested nutrients are primarily large polymers that cannot be absorbed by the intestinal cell membrane unless they are transformed into an absorbable molecular form. In addition, a coordinated interplay of GI motility and neurohormonal secretion is required to facilitate adequate digestion and absorption.
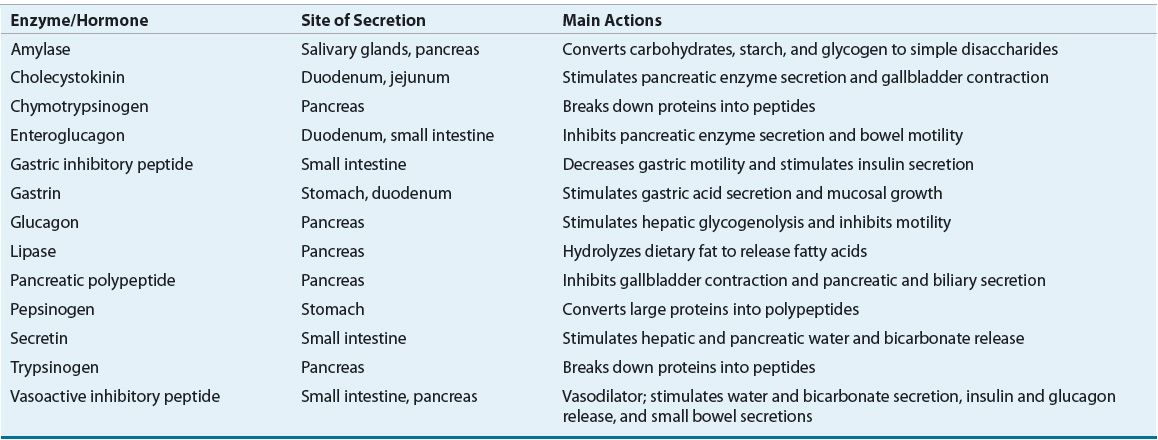
Nutrient digestion involves the complex coordination of multiple mechanical, enzymatic, and physiochemical processes.1,2 Mechanical dissolution of food occurs by chewing, then mixing and grinding the stomach contents. Food stimulates the secretion of numerous hormones and enzymes from the salivary glands, stomach, liver and biliary system, pancreas, and intestines (see Table 120-1). As food passes along the gut lumen, these hormones modulate GI motility and the secretions from subsequent organs of the digestive system. Nutrient digestion and absorption occurs within the gut lumen and is a specific function of the intestinal cell membrane, which is comprised of fingerlike projections called villi. Each individual villus is made up of epithelial cells called entero-cytes. The enterocyte surface contains special luminal projections called microvilli, which provide an increased surface area that is referred to as the brush-border membrane.
TABLE 120-1 Gastrointestinal Enzymes and Hormones
The digestion and absorption of carbohydrate, fat, and protein within the small intestine are illustrated in Figure 120-1. Carbohydrates are presented to the small intestine in either a digestible or a nondigestible form. Polysaccharides (starches) and oli-gosaccharides (sucrose and lactose) undergo enzymatic digestion within the small intestine to produce simple sugars. The simple sugars are absorbed via active and passive transport mechanisms and are eventually released into the portal vein. Polysaccharides, such as cellulose complexes and other fiber components, pass undigested to the colon, where they are digested by bacteria and enzymes to form short-chain fatty acids. Absorption of short-chain fatty acids by the colon stimulates sodium and water reabsorption, serves as an energy source, and provides nourishment to the colonic mucosa cells.
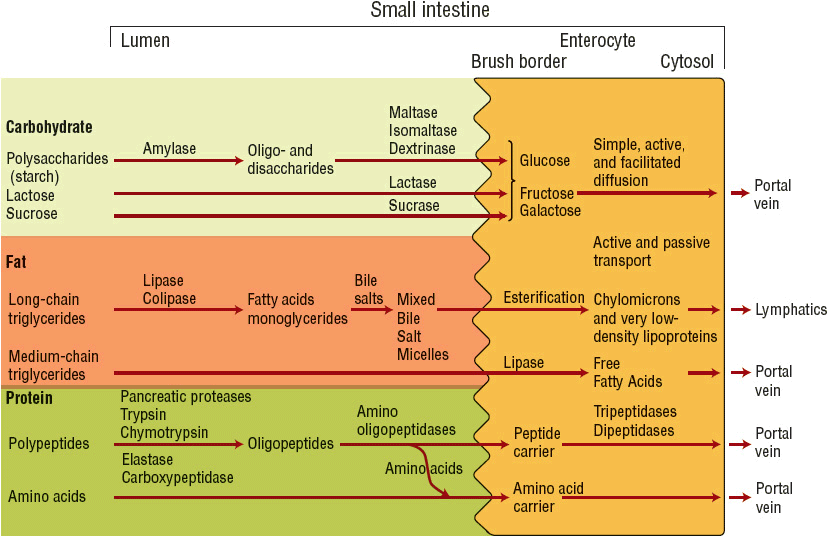
FIGURE 120-1 Schematic of carbohydrate, fat, and protein digestion.
Fat is presented to the small intestine as long-chain triglycerides. Its digestion requires pancreatic enzyme release and formation of mixed bile salt micelles, the end product, which is then absorbed across the intestinal enterocyte. Within the enterocyte, triglycerides are reesterified and packaged into chylomicrons for release into the lymphatic system. Medium-chain triglycerides (MCTs) can be absorbed intact by the mucosal membrane and are acted on by intracellular lipase within the enterocyte to release free fatty acids that pass directly into the portal vein.3
Protein is presented to the small intestine primarily as large polypeptides and to a small extent as free amino acids because of the denaturation of protein within the stomach. Luminal polypeptide digestion generates oligopeptides, which are further hydrolyzed to dipeptides and tripeptides. Absorption of peptides occurs via a peptide transport system; free amino acids are carried via specific amino acid transport systems. The carriers for the peptides are very efficient, whereas absorption of free amino acids appears to be more limited and less efficient.2
Understanding the mechanisms of digestive and absorptive physiology can greatly enhance the rational use of EN during conditions of normal or altered GI anatomy and/or function. Various circumstances may alter the efficacy of nutrient digestion and absorption. For example, the functional immaturity of the neonatal gut may lead to clinical problems associated with inadequate digestion and absorption of EN. These factors, as they relate to successful EN practice, are discussed in detail throughout this chapter.
Gut Host Defense Mechanisms
![]() Besides digesting and absorbing nutrients to maintain nutritional health, the GI tract is actively involved in defending the host from toxins and antigens by means of both immunologic and nonimmunologic mechanisms.4 These gut host defense mechanisms are collectively referred to as the gut barrier function. The gut barrier acts to prevent the spread of intraluminal bacteria and endotoxins to systemic organs and tissues. Hydrochloric acid secreted by the stomach kills most of the bacteria ingested with food. Under normal circumstances, a mucus layer coats the intestinal epithelium and thereby alters the adherence of bacteria to the cells of the GI tract but provides a favorable environment for anaerobic bacteria. Anaerobic bacteria, which normally colonize the mucus layer, aid in preventing tissue colonization by potential pathogens. Small bowel peristalsis further prevents bacterial stasis and overgrowth. The gut barrier function is also maintained by the intestinal immune system, known as the gut-associated lymphoid tissue (GALT). GALT regulates the local immune response to antigens within the GI tract. Specific immunoglobulins are secreted to kill the remaining organisms and neutralize any toxins they produce. The liver Kupffer cells help to maintain gut barrier function by clearing the portal blood of gut-derived bacteria and endotoxins. The integrity of gut barrier function may be affected negatively by numerous pathogenic insults, such as physiologic stress and ischemia, and a variety of drugs, including chemotherapeutic agents. The administration of certain probiotics can modify intestinal flora and may have beneficial effects in various disease states and patient populations by positively affecting the maintenance of gut barrier function and intestinal immune function.5,6
Besides digesting and absorbing nutrients to maintain nutritional health, the GI tract is actively involved in defending the host from toxins and antigens by means of both immunologic and nonimmunologic mechanisms.4 These gut host defense mechanisms are collectively referred to as the gut barrier function. The gut barrier acts to prevent the spread of intraluminal bacteria and endotoxins to systemic organs and tissues. Hydrochloric acid secreted by the stomach kills most of the bacteria ingested with food. Under normal circumstances, a mucus layer coats the intestinal epithelium and thereby alters the adherence of bacteria to the cells of the GI tract but provides a favorable environment for anaerobic bacteria. Anaerobic bacteria, which normally colonize the mucus layer, aid in preventing tissue colonization by potential pathogens. Small bowel peristalsis further prevents bacterial stasis and overgrowth. The gut barrier function is also maintained by the intestinal immune system, known as the gut-associated lymphoid tissue (GALT). GALT regulates the local immune response to antigens within the GI tract. Specific immunoglobulins are secreted to kill the remaining organisms and neutralize any toxins they produce. The liver Kupffer cells help to maintain gut barrier function by clearing the portal blood of gut-derived bacteria and endotoxins. The integrity of gut barrier function may be affected negatively by numerous pathogenic insults, such as physiologic stress and ischemia, and a variety of drugs, including chemotherapeutic agents. The administration of certain probiotics can modify intestinal flora and may have beneficial effects in various disease states and patient populations by positively affecting the maintenance of gut barrier function and intestinal immune function.5,6
INDICATIONS FOR ENTERAL NUTRITION
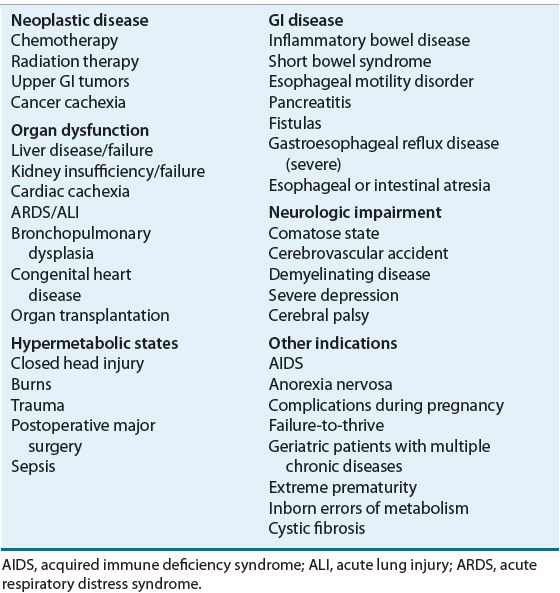
![]() The decision to initiate EN is based on a variety of factors. Suitable candidates are those who cannot or will not eat a sufficient amount to meet nutritional requirements, those who exhibit a sufficient functioning GI tract to allow the absorption of nutrients, and those in whom a method of enteral access can be safely obtained.7–9 EN may be indicated in a variety of conditions or disease states (Table 120-2). For example, patients who have neurologic disorders, such as a cerebrovascular accident, and have difficulty swallowing often require EN. Patients unable to eat because of conditions such as facial or jaw injuries, lesions of the oral cavity or esophagus, esophageal stricture, or head and neck cancer may also be candidates for EN delivered distal to the affected site. Extreme prematurity necessitates tube feeding because the suck–swallow mechanism has not yet developed sufficiently to allow safe oral intake.
The decision to initiate EN is based on a variety of factors. Suitable candidates are those who cannot or will not eat a sufficient amount to meet nutritional requirements, those who exhibit a sufficient functioning GI tract to allow the absorption of nutrients, and those in whom a method of enteral access can be safely obtained.7–9 EN may be indicated in a variety of conditions or disease states (Table 120-2). For example, patients who have neurologic disorders, such as a cerebrovascular accident, and have difficulty swallowing often require EN. Patients unable to eat because of conditions such as facial or jaw injuries, lesions of the oral cavity or esophagus, esophageal stricture, or head and neck cancer may also be candidates for EN delivered distal to the affected site. Extreme prematurity necessitates tube feeding because the suck–swallow mechanism has not yet developed sufficiently to allow safe oral intake.
TABLE 120-2 Potential Indications for Enteral Nutrition
Critically ill patients who are endotracheally intubated for mechanical ventilation represent a large percentage of patients requiring EN. Traditionally, EN in the critically ill population was regarded as supportive care designed to provide nutrients during the period of time the patient was unable to maintain oral dietary intake. Recently, the use of EN has been initiated to modulate the stress response to critical illness and improve patient outcomes. Nutrition guidelines support the initiation of EN in critically ill adults10–12 and children13 who are unable to maintain volitional intake. Some of these patients may have reduced gastric emptying caused by sepsis, GI surgery, anesthetic agents, opioid analgesics, and underlying pathology, such as diabetic gastroparesis and burns. However, successful EN can often be achieved by bypassing the stomach and placing the tip of the feeding tube beyond the pylorus into the duodenum, or preferably into the jejunum. Small bowel feeding may also be appropriate for patients with gastric outlet obstruction, those with pancreatitis, those with moderate to severe gastroesophageal reflux, or those with high risk of aspiration.
The only absolute contraindications for EN are distal mechanical intestinal obstruction12 and necrotizing enterocolitis.14 However, conditions such as severe diarrhea, protracted vomiting, enteric fistulas, severe GI hemorrhage, and intestinal dysmotility may result in significant challenges to the successful use of EN.
BENEFITS OF ENTERAL NUTRITION
The importance of maintaining nutrient delivery through the GI tract in patients without a contraindication to its use is well supported. The beneficial effects of EN, specifically in the critically ill patient, are further enhanced if EN is initiated within 24 to 48 hours of admission to an intensive care unit (ICU).10
Enteral Versus Parenteral Nutrition
Clinical studies comparing EN and parenteral nutrition (PN) in the critically ill patient demonstrate a decrease in infectious complications and thus improved outcomes with the use of EN.15–19 Infectious complications are less common with EN in part because EN supports the functional integrity of the gut by stimulating bile flow and the release of endogenous trophic agents, such as cholecystokinin, gastrin, and bile salts. Provision of enteral nutrients appears to help maintain the villous height of the intestinal mucosa and support the mass of secretory immunoglobulin A (IgA)-producing immunocytes that comprise the GALT. In the setting of critical illness or injury, adverse changes in gut permeability and gut barrier function that result in increased risk for systemic infection and multiorgan dysfunction syndrome have been noted. By supporting gut integrity, the enteral route of feeding is more likely than the parenteral route to lower the risk of infection and minimize organ failure.10
Critical reviews of available prospective randomized, controlled trials comparing EN with PN in the critically ill adult patient with an intact GI tract suggest a significant reduction in infectious complications associated with EN.10,11 Decreased infectious complications have been documented in patients with abdominal trauma, burns, severe head injury, major surgery, and acute pancreatitis. The reduced infectious complications are primarily the result of a lower incidence of pneumonia and catheter-related bloodstream infections in most of these patient populations and a decrease in abdominal abscess in trauma patients. EN is thus preferred over PN for the feeding of critically ill adult patients requiring specialized nutrition support.10,11 There are no randomized, controlled trials that compare the use of EN and PN in children.13
EN is more physiologic than PN in terms of nutrient utilization and therefore is generally associated with fewer metabolic complications, such as glucose intolerance and elevated insulin requirements.20 Enteral formulations contain both complex and simple carbohydrates, which results in slower carbohydrate absorption compared with the simple carbohydrate, dextrose, used in PN. In addition, enteral formulations that contain fiber and/or a high fat content will further slow carbohydrate absorption and decrease any elevation in blood sugar by delaying gastric emptying. This may account for better blood glucose control when carbohydrate is given via the enteral route. An additional physiologic benefit of enteral feeding is that it stimulates bile flow through the biliary tract and thus reduces the risk of developing cholestasis, gallbladder sludge, and gallstones, conditions that have been associated with long-term PN and bowel rest.21 Also, EN avoids the potential infectious and technical complications associated with the placement and use of a central venous access device required for PN. Finally, EN is less costly than PN when all factors are considered.
Early Versus Delayed Initiation
The timing of initiation of EN in the critically ill patient is of clinical significance. Initiating EN in the first 24 to 72 hours following admission appears to attenuate the stress response and may reduce disease severity and infectious complications when compared with the initiation of feedings after 72 hours.10,11 Early EN has also been associated with a decrease in the release of inflammatory cytokines and fewer alterations in gut permeability.22 Clinical studies demonstrating a decrease in infectious complications with the use of EN compared with PN in the critically ill patient initiated feeding within 24 to 48 hours of hospital admission.10,11,15,22,23 The benefits of decreased infectious complications are not apparent when the initiation of EN is delayed. A review of available studies comparing early versus delayed EN in critically ill patients showed a trend toward a reduction in infectious complications with early EN.10,11 In addition, a trend toward reduction in mortality associated with early EN has been noted.10,11,24
In critically ill patients who are hemodynamically unstable, early EN may result in gut ischemia because of poor gut blood flow and increased oxygen demand. Consequently, it is recommended that initiation of EN be delayed until the patient is fluid resuscitated and has an adequate perfusion pressure. Once this goal is achieved, often within 6 hours of hospitalization, the initiation of EN at a low administration rate is considered appropriate, along with clinical monitoring to ensure GI tolerance.25,26 Therefore, early EN (within 24 to 48 hours after hospital admission) is recommended in critically ill adult patients.10,11 Although no randomized, controlled trials have assessed early EN in critically ill children, initiation of EN within 48 to 72 hours of admission is common.13 Early initiation of EN is not warranted for the mild to moderately stressed adult patient who is otherwise well nourished. It is reasonable to delay the initiation of EN in these patients until oral intake is inadequate for 7 to 14 days.7 In the mild to moderately stressed adult patient with inadequate oral intake who is malnourished, it is unclear when to initiate EN, but most clinicians would wait no longer than 7 days.
ENTERAL ACCESS
Advances in enteral access techniques have contributed to the expanded use of EN for conditions in which PN had previously been used. In particular, improved methods of achieving jejunal access for feeding have allowed for the use of EN during the early postoperative and postinjury period when gastric motility is typically impaired. As outlined in Table 120-3, various factors influence the selection of enteral access site and device, including anticipated duration of use (short- or long-term) and whether to feed into the stomach or small bowel. Figure 120-2 illustrates the predominant enteral access options.
TABLE 120-3 Options and Considerations in the Selection of Enteral Access
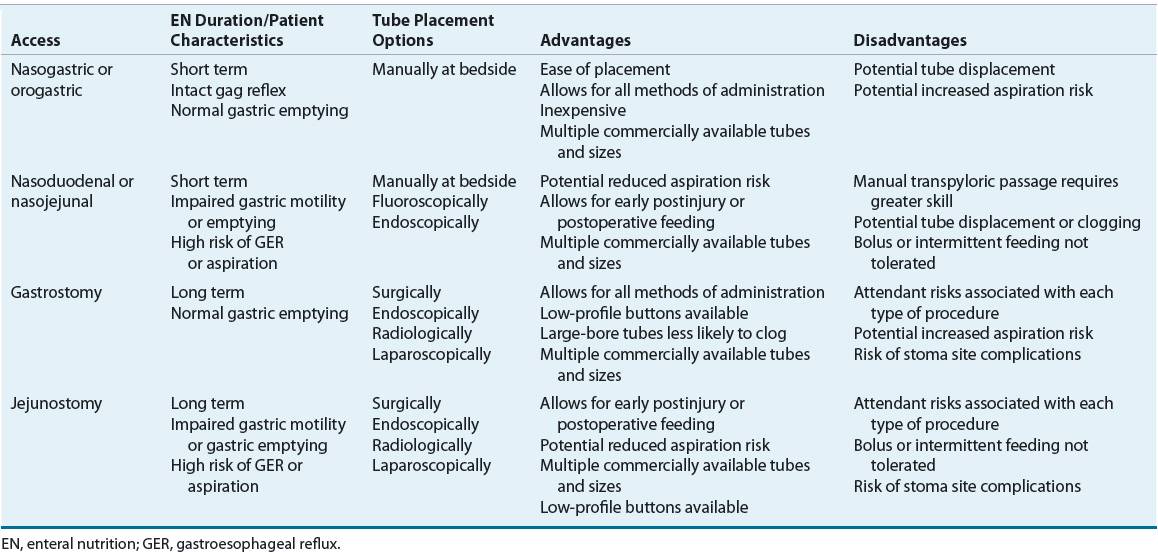
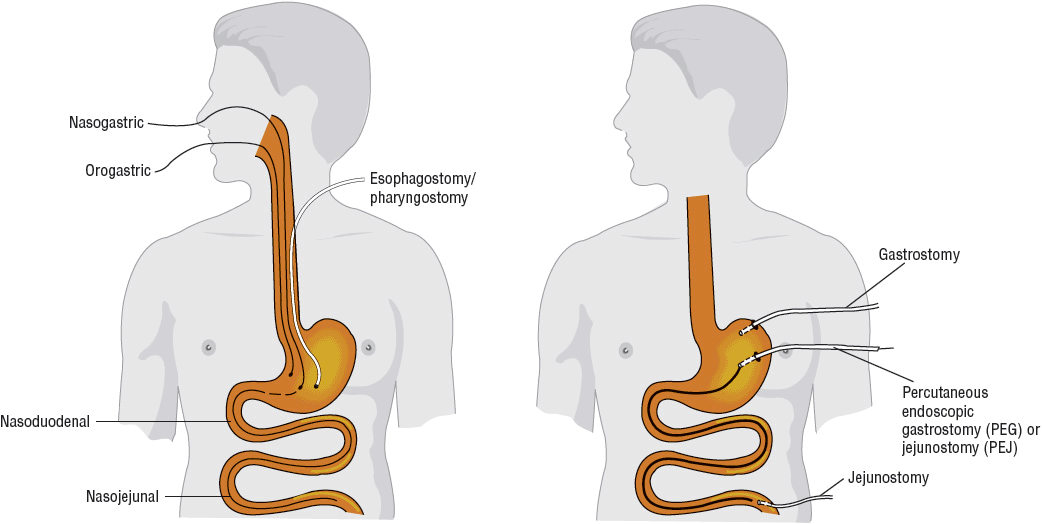
FIGURE 120-2 Access sites for tube feeding.
Short-Term Access
![]() Short-term enteral access is easier to initiate, less invasive, and less costly than the establishment of long-term access.27 The most frequently used routes for short-term enteral access are established by inserting a tube through the nose and passing the tip into the stomach (nasogastric [NG]), duodenum (nasoduodenal), or jejunum (nasojejunal). In general, these tubes are used in the hospitalized patient when the anticipated tube feeding duration is less than 4 to 6 weeks. The orogastric route is generally reserved for patients in whom the nasopharyngeal area is inaccessible or in young infants who are obligate nasal breathers. Because these routes do not require surgical intervention, they are the least invasive. The feeding tube is frequently held in place only by a piece of tape on the nose or face; therefore, it can be inadvertently pulled out relatively easily.
Short-term enteral access is easier to initiate, less invasive, and less costly than the establishment of long-term access.27 The most frequently used routes for short-term enteral access are established by inserting a tube through the nose and passing the tip into the stomach (nasogastric [NG]), duodenum (nasoduodenal), or jejunum (nasojejunal). In general, these tubes are used in the hospitalized patient when the anticipated tube feeding duration is less than 4 to 6 weeks. The orogastric route is generally reserved for patients in whom the nasopharyngeal area is inaccessible or in young infants who are obligate nasal breathers. Because these routes do not require surgical intervention, they are the least invasive. The feeding tube is frequently held in place only by a piece of tape on the nose or face; therefore, it can be inadvertently pulled out relatively easily.
NG tubes vary in diameter size and stiffness. Large-bore (≥14F) rigid NG tubes are used primarily to decompress the stomach but can also be used for feeding. There is a low incidence of clogging with these tubes, and they provide a reliable way to measure gastric residual volumes (GRVs). The major disadvantage associated with the use of these tubes is patient discomfort. Small-bore nasal tubes designed solely for feeding are available in varying lengths (16 to 60 inches [41 to 152 cm]) and diameter sizes (4F to 12F) to accommodate both pediatric (including neonates) and adult patients. The tip of the tube can be placed into the stomach, duodenum, or jejunum. These tubes consist of a lightweight, pliable silicone or polyurethane material that is more comfortable for the patient. A disadvantage of the small-bore tubes is that they may become easily occluded, often as a result of improper medication administration or tube-flushing technique.
![]() In general, the stomach is the least expensive and the least labor-intensive access site to use for enteral feeding; however, feeding into the stomach is not always tolerated. Patients with impaired gastric motility may be predisposed to aspiration and pneumonia when feedings are delivered into the stomach. Many critically ill, injured, and postoperative patients exhibit delayed gastric emptying, limiting their ability to tolerate gastric feeding. In addition, patients with diabetic gastroparesis or patients with severe gastroesophageal reflux disease or intractable vomiting are at a higher risk for aspiration of gastric contents, resulting in pneumonia. In these patients, placing the tip of the tube into the duodenum or jejunum (also referred to as transpyloric placement) has been suggested as a method to decrease risk for aspiration.12 Nasoduodenal feeding has been associated with a lower rate of vomiting and ventilator-associated pneumonia when compared to NG feeding.28 However, the evidence to support the difference in aspiration and aspiration pneumonia risk associated with gastric and small bowel feeding is inconclusive. In general, small bowel feeding may be beneficial in patients who do not tolerate gastric feeding and offers an alternative to PN.10–13 Nasoenteric feeding tubes can be inserted at the patient’s bedside by trained medical personnel. However, greater skill is required to advance the tip of the feeding tube beyond the pylorus. Several techniques have been described in the literature to help facilitate bedside placement. Variable success rates have been reported with these techniques and are largely dependent on clinician experience. Electromagnetic tube placement devices that can be used at the bedside to guide tip position into the small bowel have been shown to be safe and cost-effective for small bowel feeding tube placement.29,30 Alternatively, a variety of endoscopic and fluoroscopic techniques have been described to insert transpyloric tubes.12,27 Radiographic confirmation of appropriate tip placement should be obtained prior to use for all feeding tubes inserted by bedside techniques.7,8
In general, the stomach is the least expensive and the least labor-intensive access site to use for enteral feeding; however, feeding into the stomach is not always tolerated. Patients with impaired gastric motility may be predisposed to aspiration and pneumonia when feedings are delivered into the stomach. Many critically ill, injured, and postoperative patients exhibit delayed gastric emptying, limiting their ability to tolerate gastric feeding. In addition, patients with diabetic gastroparesis or patients with severe gastroesophageal reflux disease or intractable vomiting are at a higher risk for aspiration of gastric contents, resulting in pneumonia. In these patients, placing the tip of the tube into the duodenum or jejunum (also referred to as transpyloric placement) has been suggested as a method to decrease risk for aspiration.12 Nasoduodenal feeding has been associated with a lower rate of vomiting and ventilator-associated pneumonia when compared to NG feeding.28 However, the evidence to support the difference in aspiration and aspiration pneumonia risk associated with gastric and small bowel feeding is inconclusive. In general, small bowel feeding may be beneficial in patients who do not tolerate gastric feeding and offers an alternative to PN.10–13 Nasoenteric feeding tubes can be inserted at the patient’s bedside by trained medical personnel. However, greater skill is required to advance the tip of the feeding tube beyond the pylorus. Several techniques have been described in the literature to help facilitate bedside placement. Variable success rates have been reported with these techniques and are largely dependent on clinician experience. Electromagnetic tube placement devices that can be used at the bedside to guide tip position into the small bowel have been shown to be safe and cost-effective for small bowel feeding tube placement.29,30 Alternatively, a variety of endoscopic and fluoroscopic techniques have been described to insert transpyloric tubes.12,27 Radiographic confirmation of appropriate tip placement should be obtained prior to use for all feeding tubes inserted by bedside techniques.7,8
Long-Term Access
Feeding tubes used for short-term enteral access are usually not optimal for long-term use because of patient discomfort, complications, and mechanical failures that develop over time. Long-term access should generally be considered when EN is anticipated for longer than 4 to 6 weeks. Many techniques can be used to establish long-term enteral access, including laparotomy, laparoscopy, endoscopic and image guidance (e.g., fluoroscopy, ultrasound).12 The ability to perform the various techniques will be somewhat dependent on the expertise and facilities available within each institution. Long-term enteral access options include gastrostomy and jejunostomy tubes.
A gastrostomy is the most common type of long-term enteral access. It eliminates the nasal irritation and discomfort associated with nasoenteric feeding tubes and inadvertent removal is uncommon. In addition, because feeding gastrostomies use large-bore tubes, clogging is less of a problem. The most common technique for placement is the percutaneous endoscopic gastrostomy (PEG). It is minimally invasive and can be performed safely and cost-effectively in an endoscopy suite or at the bedside using conscious sedation and local anesthesia. Young children, however, will usually require general anesthesia for the procedure. Gastrostomy tubes are available in various sizes (12F to 28F; 1 to 4.5 cm shaft lengths), material (e.g., silicone, polyurethane), and have different retention mechanisms. Since smaller-diameter tubes are prone to more frequent occlusion and dysfunction, the larger diameter size is usually preferred. For patient convenience and comfort, a low-profile skin-level gastrostomy device may be placed in 2 to 3 months, once the gastrostomy tract has matured, if this type of device was not placed initially. This “gastric button” consists of a short, silicone, self-retaining conduit with either a mushroom tip or a balloon at the internal end and a one-way valve and small flange at the skin surface. Because this averts the external tube presence, it tends to be preferred in children or ambulatory adults who are receiving intermittent feedings. The exit site of all gastrostomies requires general stoma care to prevent inflammation and infection. Routine replacement of the gastrostomy tube at defined intervals (usually 3 to 6 months) is a standard of practice of many clinicians to prevent failure of the retention mechanism that can occur over time.12
In patients with a functional bowel but impaired gastric motility, pancreatitis, or who otherwise do not tolerate gastric feeding and require long-term enteral access, a jejunostomy may be an appropriate option.27 Various endoscopic and fluoroscopic techniques are available for direct jejunostomy placement. A surgically placed jejunostomy may be an option if the patient requires a laparotomy or laparoscopy for other reasons. For patients who require small bowel feeding with simultaneous gastric decompression, a gastrojejunal tube may be placed utilizing various endoscopic, fluroscopic, and surgical techniques.12,27 Because jejunostomies use smaller-bore tubes, occlusion occurs more commonly than with gastrostomy tubes. Gastrojejunostomy tubes are often replaced every 3 to 6 months to prevent occlusion.
Pharyngostomies and esophagostomies are invasive because the tube is located in the neck and passes through the skin into the pharynx or esophagus, respectively. They are rarely performed because of the high complication rate and extreme difficulty associated with their maintenance care.31 However, they may be used in patients with head and neck malignancies and when placement of a gastrostomy or jejunostomy tube is not possible due to GI obstruction.
There are ethical implications regarding determination of appropriate candidates for long-term feeding tube placement.12,31,32 Because a gastrostomy is relatively easy to place and many patients, families, and nonspecialist clinicians overestimate the benefits of EN, it is prone to inappropriate use. In certain patient populations, such as those with end-stage cancer or advanced dementia, the placement of a gastrostomy is controversial. Artificial nutrition and hydration (ANH) has not been shown to promote the healing of pressure ulcers, increase patient comfort or functional status, or prolong survival when compared to hand feeding in patients with advanced dementia.32 From a clinical standpoint, ANH does not increase a patient’s comfort or improve nutrition parameters of most terminally ill people and can result in medical complications.33 Placement of a feeding tube resulted in a higher 1-year mortality rate in a group of 5,266 nursing home residents with dysphagia.34 Evaluation by a multidisciplinary team is warranted for patients near end of life to establish whether the benefit outweighs the risk of feeding tube placement.8,32
ADMINISTRATION METHODS
EN may be administered by continuous, cyclic (continuous rate over a portion of the day), intermittent (infused over 20 to 60 minutes), or bolus (generally given in 5 to 10 minutes) methods and may be accomplished by syringe, gravity, or pump-controlled techniques. The method of delivery depends on the location of the tip of the feeding tube, the patient’s clinical condition and intestinal function, the environment in which the patient resides, and the patient’s tolerance to the tube feeding.
Continuous
Pump-assisted continuous administration of EN is generally the method of choice for feeding patients who are critically ill, have poor glycemic control, are being fed via jejunostomy tube, or have demonstrated intolerance to intermittent or bolus feeding.9,35 When EN is delivered into the small intestine, the continuous method is preferred because it is associated with enhanced tolerance. The rapid delivery of feeding into the small intestine, especially hyperosmotic formulations, may contribute to abdominal distension, cramping, hyperperistalsis, and diarrhea. Therefore, conversion to intermittent or bolus administration is not recommended for those with jejunostomy tubes.
The delivery system for continuous administration generally includes a feeding reservoir or bag attached to an extension set that is connected to a pump. The delivery system is then attached to the patient’s enteral access tube. Continuous administration may increase nursing time because routine checks are needed, but this disadvantage is offset by the improved tolerance. For adults, target EN administration rates generally range from 50 to 125 mL/h, although higher rates have been used without complications. In infants and children, goal administration rates vary with age and weight and should be sufficient to meet caloric needs while maintaining good GI tolerance. The primary disadvantage to this method of administration is the cost and inconvenience associated with the pump and administration sets. In the home care setting, battery-operated ambulatory enteral pumps are available to allow the patient greater mobility.
Cyclic
A patient who is not eating well during the day because of complaints of fullness and lack of appetite may benefit from a trial of cyclic EN, in which the enteral feeding is administered only at night. In addition, EN administration only overnight will free the patient from the pump during the day and allow for greater mobility. This increased mobility may be particularly useful for the home patient or patient requiring rehabilitation. Because a pump controls the rate of administration, this method may be used in patients with either gastric or small bowel access.
Bolus
The bolus administration of EN is commonly used for patients in long-term care settings who have a gastrostomy. This administration technique involves the delivery of the enteral feeding formulation over 5 to 10 minutes. Essentially, the only equipment needed is a syringe to instill the feeding volume into the tube. Depending on the patient’s nutritional requirements, an instillation volume of 240 to 500 mL is generally used and repeated four to six times daily. From a convenience standpoint, it is generally preferable to adjust the bolus volume in increments of the feeding formulation container size (usually 240 to 250 mL). Bolus volumes given to infants and children vary with age and weight (usually 30 to 240 mL) and should be sufficient to meet the calorie needs of most patients. In neonates, the bolus regimen is usually begun with an every 3-hour schedule; as the child grows, feedings may be given less frequently.36 In patients with duodenal or jejunal access, bolus delivery may result in cramping, nausea, vomiting, aspiration, and diarrhea. Bolus administration also should be avoided in patients with delayed gastric emptying and in patients who are at high risk of aspiration.
Intermittent
If a patient is experiencing intolerance to bolus administration over 5 to 10 minutes, it may be helpful to administer the prescribed volume over a longer time period, generally 20 to 60 minutes. For this method, the desired volume of feeding formulation is emptied into a reservoir bag or container and administered by an enteral pump or via gravity drip using a roller clamp. The bolus method of administration is more consistent physiologically with normal eating patterns than the continuous method. One study in infants demonstrated that normal gallbladder emptying did not occur with continuous feedings but was present in those infants receiving bolus feedings.37 Thus, those patients who need long-term EN and PN, especially children, may benefit when this approach is used because it may minimize the development of cholestatic liver disease.
INITIATION AND ADVANCEMENT PROTOCOL
Guidelines for the initiation and advancement of enteral feeding formulations vary greatly and are primarily tailored to patient tolerance. The typical recommendation for continuous EN administration for adults is to start at 20 to 50 mL/h and advance by 10 to 25 mL/h every 4 to 8 hours until the desired goal is achieved. For intermittent administration, the typical recommendation is to start with 120 mL every 4 hours and advance by 30 to 60 mL every 8 to 12 hours.8,35 In children, the recommendation for continuous administration is initiation at a rate of 1 to 2 mL/kg per hour (no more than 25 to 30 mL/h) or 2 to 4 mL/kg per bolus (no more than 30 to 90 mL) with advancement by similar amounts every 4 to 24 hours. In premature infants, feedings may be initiated at lower rates usually 10 to 20 mL/kg per day and advanced by similar rates daily. Schedules for progression of tube feeding from initial to target rates are important and may influence tolerance. If the protocol is too conservative, it may take an excessively long period of time to reach nutrient goals. The practice of diluting enteral feeding formulations is not routinely recommended unless necessary to increase fluid intake.8,35 The development of an EN protocol within an institution that outlines initiation and advancement criteria may be a useful strategy to optimize achievement of nutrient goals.10,11 Such a protocol should allow nursing to advance the rate (e.g., 25 mL/h every 4 hours until the goal rate is achieved) based on GI tolerance. Clinical signs of intolerance include abdominal distension, abdominal cramping, high GRVs, aspiration, and diarrhea.
ENTERAL FEEDING FORMULATION SELECTION
Historically, enteral formulas were created to provide essential nutrients. Over the years, enhancements have been made to meet specific patient needs and improve tolerance. For example, nutrient composition has been enhanced by changing the content of the amino acids (e.g., glutamine and arginine), changing the omega-3 polyunsaturated fatty acid content, and adding RNA to enhance immune function and improve therapeutic outcomes. These specific nutrients have been called nutraceuticals or pharmaconutrients because of the intent to use them to modify disease processes and improve clinical outcomes. Currently, enteral feeding formulations are categorized by the FDA as medical foods.8 They are considered components of supportive care and are simply regulated to ensure sanitary manufacture. Unfortunately, they are not subject to rules governing health claims, and promotion of medical foods for therapeutic intent is currently not regulated by the FDA.38
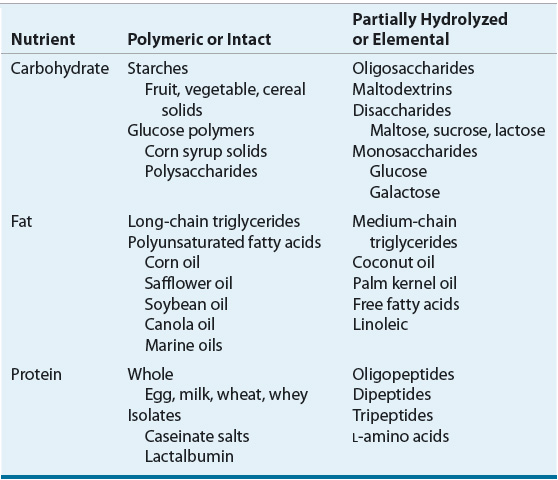
The macronutrient content of enteral formulas (namely, protein, carbohydrate, and fat) varies in nutrient complexity (Table 120-4). Nutrient complexity refers to the amount of hydrolysis and digestion a substrate requires prior to intestinal absorption. Polymeric or intact substrates are of similar molecular form as the foods we eat. Enteral formulas that contain partially hydrolyzed or elemental substrates are characterized as elemental or defined-formula diets. The caloric contribution of each of the macronutrients is as follows: carbohydrates, 4 kcal/g (17 kJ/g); protein, 4 kcal/g (17 kJ/g); and fat, 9 kcal/g (38 kJ/g).
TABLE 120-4 Enteral Formula Nutrient Complexity