Endovascular Interventions for Deep Venous Thrombosis
Erin H. Murphy
Mihaiela Ilves
Frank R. Arko
Introduction
Despite the initiatives to decrease the incidence of deep venous thrombosis (DVT) with the routine use of chemical and mechanical thromboprophylaxis regimens, DVT remains the third most common cardiovascular disease in the USA. Each year 600,000 new cases are diagnosed in the United States. Associated morbidity and mortality is high, underscoring the need for early diagnosis and intervention.
Medical management with anticoagulation has been the mainstay of therapy and is effective in preventing clot propagation, pulmonary emboli (PE), and death. This treatment alone is sufficient for isolated calf vein DVT with excellent clinical outcomes.
Patients with more proximal iliofemoral DVT, however, while benefiting from the short-term benefits of anticoagulation, are subject to long-term morbidity not addressed by anticoagulation alone. These patients have more complete obstruction, higher clot burden, and often have underlying venous lesions which result in slower or incomplete resolution of venous obstruction. Permanent valvular damage from prolonged venous obstruction results in symptoms of post-thrombotic syndrome in up to
90% of patients with iliofemoral DVT despite adequate anticoagulation. Symptoms range from venous hypertension to severe venous claudication and ulceration. Additionally, patients with iliofemoral DVT treated with anticoagulation alone experience significantly higher rates of DVT recidivism both during and after treatment.
90% of patients with iliofemoral DVT despite adequate anticoagulation. Symptoms range from venous hypertension to severe venous claudication and ulceration. Additionally, patients with iliofemoral DVT treated with anticoagulation alone experience significantly higher rates of DVT recidivism both during and after treatment.
Intervention for Proximal Dvt
More aggressive initial attempts at early thrombus removal have been advocated for proximal DVT to prevent debilitating long-term sequelae. Early clot removal allows restoration of venous flow and preservation of valvular competence, preventing the development of post-thrombotic syndrome.
The benefits of early clot removal were first demonstrated with surgical venous thrombectomy. This technique was associated with improved long-term venous patency and a substantially reduced incidence of post-thrombotic syndrome. However, the invasive nature of this procedure prevented widespread clinical adoption. Systemic thrombolysis, introduced as a less invasive means of early thrombus removal, demonstrated similar improvements in venographic and clinical outcomes but failed to improve clinical utility secondary to unacceptably high bleeding complications.
The advent of advanced endovascular techniques has resulted in a renewed interest in interventions for iliofemoral DVT. These treatments are safer and result in more effective clot removal than earlier strategies, and offer the additional advantage of allowing intraoperative diagnosis and treatment of underlying venous pathology. Treatment of underlying venous stenoses, which exist in the majority of patients with proximal DVT, aids in thrombus resolution and helps prevent disease recurrence. Endovascular intervention for DVT should be considered in all patients with proximal lower extremity DVT and a reasonable life expectancy.
Patients presenting with suspected lower extremity DVT should complete diagnostic evaluation prior to intervention to confirm diagnosis, determine full thrombus extent, and evaluate for underlying pathology contributing to venous obstruction. Though venography remains the gold standard for diagnosis and assessment of DVT, this is both invasive and impractical as a standard diagnostic tool and is now reserved as a confirmatory study completed at the time of intervention.
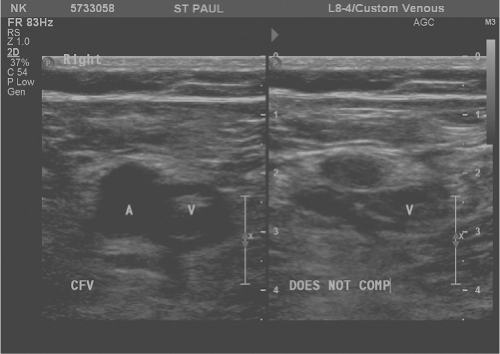
Lower extremity duplex ultrasound can accurately diagnose DVT and is minimally invasive, low risk, convenient, and cost-effective. Ultrasonographic evidence of DVT includes non-compressible or partially compressible venous segments, continuous venous flow patterns, and the absence of normally phasic flow variation (Fig. 1). The sensitivity and specificity of duplex ultrasonography in diagnosing proximal lower extremity DVT is 95% and 97%, respectively.
Thrombophilia evaluation has been suggested in patients <50 years old, in absence of additional risk factors for DVT, in a family history of thromboembolic disorders, in unusual thrombus location, or in recurrence of DVT. These patients should be screened for protein C or S deficiency, antithrombin deficiency, factor V Leiden, protein C resistance, prothrombin gene mutation 2021A, and antiphospholipid antibody syndrome. Diagnoses of thrombophilia disorders are important in the determination of duration of anticoagulation.
Therapeutic anticoagulation with unfractionated heparin or low-molecular weight heparin should be administered once diagnosis is established and blood is drawn for the thrombophilia evaluation. Anticoagulation is administered through the operation until at least 6 months postoperatively or until a longer time period determined by the treating physician for treatment of underlying thrombophilia disorders.
Endovascular Management of Dvt
Though multiple techniques and devices are available for endovascular DVT intervention, the majority of available treatment options may be separated by mechanism of action and include the following: catheter-directed thrombolysis (CDT), ultrasound-accelerated thrombolysis, and percutaneous mechanical thrombectomy (PMT).
General Considerations
Percutaneous access for endovascular interventions is most often achieved in the vein distal to the occluded segment. For isolated iliac DVT, an ipsilateral common femoral puncture is most appropriate. Alternatively, a retrograde approach from the contralateral femoral vein may be used for isolated iliac and femoral vein DVT. More commonly, however, patients present with more extensive iliofemoral or iliofemoral popliteal thrombosis, in which case access is best obtained from the ipsilateral popliteal vein with the patient in prone position. Ultrasound guidance should be considered for access of the popliteal or tibial veins and for any access obtained while the patient is fully anticoagulated. Further, a micropuncture technique with a 22-gauge needle and 0.014 in. guidewire may minimize bleeding complications and vessel wall trauma (Fig. 2).
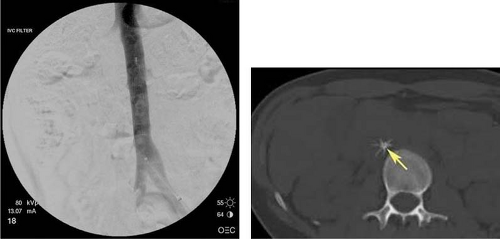
Initial venogram should be used to confirm diagnosis and thrombus extent. The thrombus then is crossed with a guidewire to facilitate catheter or device positioning. The use of retrievable inferior vena caval (IVC) filters during CDT or PMT may be prudent for prevention of peri-procedural pulmonary embolization as clot is disrupted. In an animal model, IVC filters were shown to decrease the incidence of angiographically diagnosed PE after mechanical thrombolysis of DVT. Further, Thery et al. have demonstrated that 31% of the patients (41/132) undergoing thrombolysis for lower extremity DVT after placement of retrievable IVC filters had significant thrombus in the filter at the end of the procedure. More importantly, no patients in this study suffered a PE. Subsequent study confirmed these results, demonstrating a 40% incidence of thrombus debris in implanted filters following mechanical thrombectomy.
It is our standard practice, and recommendation, to use retrievable IVC filters prior to thrombolysis or mechanical thrombectomy (Fig. 3). In over 30 patients treated at our institution with IVC filters, mechanical thrombectomy, and CDT, there have been no occurrences of symptomatic PE, no filter-related complications, and all IVC filters have been successfully removed. Other authors have reported similar success with IVC filters in this setting.
Filters used for PE prophylaxis in the setting of CDT or PMT should be placed in standard fashion below the renal veins prior to thrombectomy. In addition, access for filter deployment should be planned such that the endovenous delivery route is free of thrombus. Often, filters may be placed from the contralateral femoral vein in a percutaneous fashion. However, extension of DVT into the IVC may require filter placement from the internal jugular vein to avoid thrombus disruption during placement.
Timing of filter retrieval is largely surgeon’s preference. It is generally accepted that filters may be removed at the end of the procedure if completion venogram reveals no substantial clot burden. However, filter thrombus or persistent post-procedural contraindication for anticoagulation may require the filter to be left in place. In our experience, these filters may then be removed without complications at a later date.
Endovascular Treatment Options
Selection of an endovascular treatment strategy for lower extremity DVT is largely based on surgeon’s preference and institutional device availability. All available devices have been shown to be both safe and effective for proximal DVT treatment. Nonetheless, differences do exist between the techniques, which may make them subtly more or less suitable for an individual patient.
Catheter-Directed Thrombolysis (Cdt)
Catheter-directed thrombolysis involves the direct infusion of lytics at the location of venous thrombosis, thus limiting systemic lytic exposure. To achieve this localized drug delivery, a small infusion catheter is placed at the proximal extent of the thrombus and secured in place. Thrombolytics may then be slowly administered while monitoring
the patient in the intensive care unit. A variety of thrombolytic agents may be used including: Urokinase (ImaRx Therapeutics, Tucson, AZ), tissue plasminogen activator (Activase, Genentech, South San Francisco, CA), recombinant tissue plasminogen activator (Retavase, PDL BioPharma, Fremont, CA), or tenecteplase (Genentech). Repeat venography and assessment of clot lysis is performed every 24 hours until complete lysis is achieved.
the patient in the intensive care unit. A variety of thrombolytic agents may be used including: Urokinase (ImaRx Therapeutics, Tucson, AZ), tissue plasminogen activator (Activase, Genentech, South San Francisco, CA), recombinant tissue plasminogen activator (Retavase, PDL BioPharma, Fremont, CA), or tenecteplase (Genentech). Repeat venography and assessment of clot lysis is performed every 24 hours until complete lysis is achieved.
Success with this technique has been widely reported with long-term patency and reduced incidence of post-thrombotic syndrome correlating with the degree of thrombolysis achieved. A significant disadvantage of this technique lies in the need for a prolonged ICU stay during drug infusion which often ranges from 36 to 72 hours. The amount of lytics used during infusion also translates into expensive drug costs. Lastly, significant bleeding complications are still reported with this technique including insertion site bleeding in up to 45%, transfusion requirements in up to 22%, and intracranial hemorrhage in up to 3%. CDT is often best suited as an adjunct to other techniques limiting lytic times, drug dosages, and bleeding complications.
Ultrasound-Accelerated Thrombolysis—Ekos Endowave and Endosonic
The EKOS EndoWave and EndoSonic systems use low-power, high-frequency ultrasound (2.2 MHz) in combination with CDT to achieve clot disruption. The device consists of an infusion/aspiration catheter, an ultrasound core wire, and a drive unit (Fig. 4). The catheter, available in treatment lengths of 6 to 50 cm, contains a central lumen which accommodates the 0.035-ultrasound core wire. The catheter is positioned over the guidewire so that it extends though the thrombosed venous segment. The guidewire is then exchanged for the ultrasound core wire which is left in place. The catheter and wire are secured. The three separate drug infusion lumens, which are arranged in a triangular distribution around the central lumen, are primed with unfractionated heparin. The thrombolytic agent of choice is administered through each catheter and infused out through micropores located along the length of each of the three catheters. During continuous lytic infusion, the control unit is activated transmitting ultrasound energy via the central core wire to the catheter and thrombus. Normal saline is infused through the central lumen continuously during the procedure to dissipate heat production. The drive unit automatically reduces power as flow is restored, as gauged by thermocouples in the three catheters which monitor temperature and flow patterns. The procedure is continued until complete lysis is achieved.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree