Klaus Mönkemüller
DEFINITION
■ Barrett esophagus (BE) is a strong risk factor for esophageal adenocarcinoma.1
■ The annual risk of BE progression to adenocarcinoma ranges from 01.12% to 0.61%.1,2
■ The traditional treatment of choice for “resectable” esophageal adenocarcinoma is esophagectomy.
■ However, surgical resection is still associated with significant mortality and morbidity, even in high-volume centers and especially in elderly or poor surgical candidates.3
■ Thus, during the last two decades, patients with early cancer or those with high-grade dysplasia have been successfully treated with endoscopic resection methods.4,5
■ The most common method used is endoscopic mucosal resection (EMR) or “mucosectomy.”4,5
DIFFERENTIAL DIAGNOSIS
■ The differential diagnosis of mucosal neoplasia of the distal esophagus is narrow. The most common malignant neoplasia of the distal esophagus is adenocarcinoma, followed by squamous cell cancer.4,5
■ Proximal stomach cancer such as cardiac or fundic adenocarcinoma extending into the esophagus may be difficult to differentiate from distal esophageal adenocarcinoma.
■ Submucosal tumors such as gastrointestinal (GI) tumors, spindle cell tumors, lipoma, and leiomyoma are easily differentiated from mucosal lesions as these tumors generally have a normal overlying mucosa.
PATIENT HISTORY AND PHYSICAL FINDINGS
■ Most patients with Barrett’s neoplasia do not have any specific symptoms and BE is discovered incidentally during an upper GI endoscopy performed for the evaluation of gastroesophageal reflux symptoms, dyspepsia, or abdominal pain.6
■ However, Barrett’s neoplasia is more common in patients with the following characteristics: male, central obesity, Caucasian, age older than 50 years, tobacco use, and chronic gastroesophageal reflux disease (GERD).6
■ Although reflux of acid is a common occurrence in BE, a high proportion of BE patients deny a history of reflux symptoms.7
■ Therefore, any patient undergoing upper endoscopy should be carefully investigated for columnar-lined epithelium and intestinal metaplasia of the distal esophagus, with special attention to patients with the risk factors mentioned earlier.
IMAGING AND OTHER DIAGNOSTIC STUDIES
■ Radiologic studies do not play a role in the evaluation of BE unless there is a stricture present (benign or malignant). In this instance, a barium esophagogram can be helpful to determine the length and characteristics of the stenosis.
■ Although computed axial tomography is useful to evaluate for lung metastasis and mediastinal and perigastric lymph node involvement in esophageal adenocarcinoma, its role in patients with early Barrett neoplasia is negligible, as most of these patients have local disease, which does not extend beyond the submucosa of the esophagus.
■ Patients with Barrett’s cancer invading the submucosa (sm1) have a very low risk of lymph node metastasis and thus are ideal candidates for EMR.4–6
■ In the past, endoscopic ultrasound (EUS) was used to determine the depth of GI layers involvement in early Barrett neoplasia.8
■ However, the sensitivity and specificity of EUS for early Barrett neoplasia are low and the results can be misleading.8
■ The mainstay of diagnosis of BE is with light endoscopy, ideally using high-definition endoscopes and equipment.9
■ It is very important to measure the proximal distance of extension of cylindrical-type epithelium into the esophagus. The extent of BE segment should be defined using the Prague C & M classification including the length of the circumferential segment (C) and the maximal extent of the BE segment (M).10
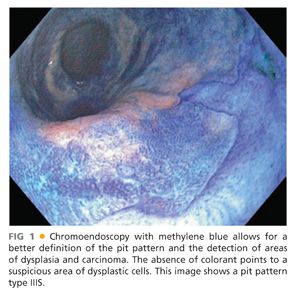
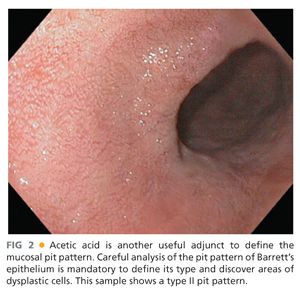
■ The use of various staining agents and dyes (chromoendoscopy) such as methylene blue (FIG 1) and indigo carmine or agents such as acetic acid (FIG 2) enhances the mucosal pit pattern analysis (see the following text) and thus facilitates the detection high-grade dysplasia and carcinoma.9
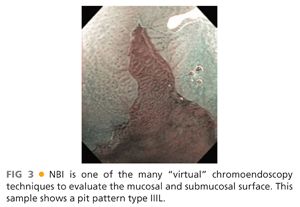
■ Other imaging modalities that might help in delineating BE are narrow band imaging (NBI) (FIG 3), autofluorescence imaging, and confocal laser endoscopy.
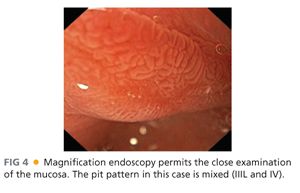
■ Magnification endoscopy is also important to characterize the mucosal surface and pit pattern (FIG 4).
■ The most widespread classification used to categorize the pit pattern in BE is the Endo classification (adapted from S. E. Kudo).11
■ This classification categorizes the opening of the pits into round (I), stellar or asteroid (II) (see FIG 2), tubular elongated (IIIL), tubular short or round (IIIS) (see FIG 1), gyrus or sulcus branched (IV) (see FIG 4), and irregular or amorphous (V) (FIG 5).
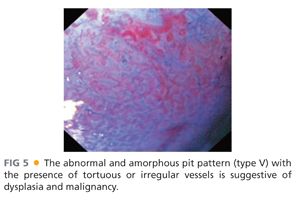
■ Types I, II, and III pit patterns are “benign,” whereas pit pattern types IV and V are more commonly present in advanced neoplasia or carcinoma.
■ Chromoendoscopy is performed to aid in obtaining directed (i.e., targeted) biopsies prior to mucosectomy to define the precise extent of neoplastic involvement.
■ Targeted biopsies are obtained from visible abnormalities, followed by four-quadrant biopsies of every 1 to 2 cm of the BE segment (Seattle protocol; see Chapter 10) and these should be reviewed by a dedicated GI pathologist.
ENDOSCOPIC MANAGEMENT
■ The two most important indications for EMR of Barrett’s neoplasia are diagnostic and therapeutic.5,6,12
■ EMR can be generally attempted if the lesions are smaller than 20 mm in diameter.
■ Endoscopic submucosal dissection (ESD) should be reserved for larger lesions.
■ However, ESD is a technique that has been mainly used for early squamous cell cancer of the esophagus. The results of ESD for Barrett neoplasia are still suboptimal.
Preoperative Planning
■ All patients should undergo a thorough history and physical examination and be classified based on the American Society of Anesthesiologists (ASA) score.
■ Interventions in patients with ASA score greater than or equal to 4 are not deemed beneficial, as the risk of harm outweighs the potential benefits.
■ Obtaining routine laboratory data such as white blood cell and platelet count, bleeding studies (partial thromboplastin time [PTT]/international normalized ratio [INR]), and creatinine and electrolytes is mainly indicated in multimorbid patients or those taking medications that specifically affect any organ or system (e.g., anticoagulant, diuretics).
■ Management of anticoagulants preoperatively should be based on the guidelines established by the American Society for Gastrointestinal Endoscopy.13
■ In patients with high-risk cardiovascular conditions on warfarin, it is important to bridge this period with the use of low-molecular or fractionated heparins.13
■ Prophylactic antibiotics are not recommended before EMR.14
■ The standard of care is the administration of conscious sedation.4,5,12,15
■ Exceptions include patients who ingest narcotics or benzodiazepines, multimorbid patients, or heavy alcohol drinkers. For these groups of patients, we prefer monitored anesthesia care (MAC), use of nurse-assisted propofol sedation (NAPS), or general anesthesia (GA).
■ We want to emphasize that a carefully performed EMR in expert hands should take less than 15 minutes.
■ When performing an EMR, the bulk of time is usually in recognizing and characterizing the lesion. The resection per se is a focused procedure that should be targeted, efficient and efficacious.
Positioning
■ The patient is placed in the left lateral decubitus position and prepared as for a routine upper endoscopy.



