Endometriosis
Clinical Features of Endometriosis
Intrinsic Abnormalities of Endometriotic Tissue
Etiologic Factors in Endometriosis
Morphologic Features of Endometriosis
Considerations at Specific Anatomic Locations
Clinical Classification of Endometriosis
Endometriosis Presenting as a Benign Solid Tumor
Introduction
Endometriosis is the condition in which endometrial tissue, composed of both endometrial-type glandular epithelium and stroma, is found at sites outside the uterine cavity. In the past, adenomyosis and endometriosis were linked by a common terminology; the former was referred to as ‘endometriosis interna’ and the latter as ‘endometriosis externa.’ The two conditions are distinct,1 however, with different symptoms and different epidemiologic and etiologic patterns: adenomyosis results from invagination of the basal endometrium lining the endometrial cavity into the uterine wall (see Chapter 20); endometriosis in most cases develops from endometrium that has implanted in the peritoneal cavity after retrograde transmission from the uterus through the fallopian tube.
Clinical Features of Endometriosis
Endometriosis is almost exclusively a disease of women in their reproductive years. It is currently the third leading cause of gynecologic hospitalization in the United States.2 Its true incidence is unknown, although most reports estimate that it occurs in about 4–13% of all women of reproductive age,3,4 25–50% of infertile women,5–7 5–25% of those admitted for pelvic pain, 50% of teenagers with intractable dysmenorrhea,3 and up to 7% of those admitted with pelvic masses. To determine the true incidence would require use of the most sensitive diagnostic test, laparoscopy, on a population of unselected premenopausal asymptomatic women. Clearly, such a prospective trial will never be performed.
The diagnosis is frequently made by laparoscopy in the late twenties or early thirties, and not infrequently in adolescence.8 In the past, when diagnosis was based primarily on symptoms, patients tended to be older at the time of diagnosis. The condition has not been reported before puberty, which is not surprising, as the sex steroid hormones needed for endometrial growth are not sufficiently abundant. Endometriosis may also occur after menopause, mostly in postmenopausal women taking exogenous hormones.9 In the minority who have not, other hormonal sources that are continuous and can sustain the process of endometriosis should be sought. One example is peripheral conversion of androgens in obese women.
Racial differences in the incidence of endometriosis, although once thought to be important, have not been confirmed in studies more adequately controlled for childbearing patterns and socioeconomic and other relevant factors.10 Women with endometriosis are generally of lower parity than non-sufferers. The relationship between endometriosis and infertility is almost certainly a vicious cycle. The hormonal milieu in a woman who does not achieve pregnancy encourages the development of endometriosis. Once endometriosis develops, its presence contributes to the infertile state and the circle is established. Conversely, pregnancy often has a beneficial effect on the disease.
Most women with endometriosis present with secondary dysmenorrhea, dyspareunia, pelvic pain, or infertility. However, only about 5% have all four major symptoms. A small number of women with endometriosis develop a pelvic mass or, occasionally, ascites.11 Many women with endometriosis are also asymptomatic. In one study two-fifths of the women in whom endometriosis was discovered during laparoscopic tubal ligation had no symptoms.12
The amount and character of the pain that the patient experiences correlate poorly with the actual extent of disease found.13 Local microenvironment and physical effects seem to be more important factors than the anatomic location and extent of the endometriosis.14 Production of cytokines and prostaglandins by the stromal cells of endometriosis has been suggested as a mechanism for stimulation of adjacent glands, and pain.15 This is supported by studies showing that aromatase inhibitors such as letrozole, which diminish paracrine stromal production of estradiol and prostaglandins, can be effective in reducing the symptom of pain.16
Distribution of Endometriosis
The sites where endometriosis is most commonly found depend greatly on whether the diagnosis rests on clinical or histologic findings. In descending order of frequency, the two most frequent sites based on clinical findings alone are the uterosacral ligaments and ovaries (Table 22.1).17 In most series, these two sites are each affected in over 60% of instances (due to multisite involvement). Most other sites are in the pelvis and include the pouch of Douglas, pelvic peritoneum, uterine surface, and fallopian tubes. The frequency of involvement ranges from 5% to 20%.
When tissue samples are selected from clinically suspicious areas, pathologic diagnosis is necessarily biased by clinical appearance. Additionally, endometriosis is a common incidental finding in bilateral salpingo-oophorectomy specimens (with or without hysterectomy) removed for unrelated reasons. Thus, in contrast to clinical series, the ovary is the principal tissue where the pathologist sees endometriosis (36% of specimens) (Table 22.2).18 The fallopian tube, uterine serosa, and cul-de-sac each account for 6–14% of biopsy-proven specimens. The uterosacral ligaments are rarely biopsied, which explains why this site accounts for <2% of biopsy-proven endometriosis.
Table 22.2
Location of Endometriosis Based upon Biopsy Findings18
| Location | Frequency (%)* |
| Ovary | 36 |
| Fallopian tube | 14 |
| Uterine serosa | 12 |
| Cul-de-sac | 6 |
| Cervix | 3 |
| Colon | 3 |
| Peritoneum | 3 |
| Appendix | 2 |
| Broad ligament | 2 |
| Pelvis | 2 |
| Uterosacral ligament | 2 |
| Vagina | 2 |
| Abdominal wall | 1 |
| Bladder | 1 |
| Fibrous tissue | 1 |
| Parametrium | 1 |
| Rectum | 1 |
| Small intestine | 1 |
| Other sites | 7 |
Epidemiology of Endometriosis
Several risk factors have been identified that are associated with the development of endometriosis. Some evidence suggests that there may be a genetic basis to endometriosis as it occurs far more commonly in monozygotic than dizygotic twins.19 The common features that appear to increase the risk of endometriosis relate to an increased exposure to menstruation, i.e., longer duration of flow or higher volumes of retrograde menstruation in states where estrogenic stimulation is maintained.20,21 It is also frequent in women with cervical stenosis. The disease is more common where there is reduced parity, which reflects delayed childbearing, and is infrequent among multiparous women.21 The risk is reduced for women using concurrent oral contraceptive medication, but not for such non-hormonal methods as intrauterine devices or diaphragms.22 The risk increases after the use of the oral contraception medication is discontinued.
The occurrence of endometriosis relates also to increasing age, peaking at ages 40–44 years.22 This may reflect increased numbers of menstrual cycles that have occurred before menopause has begun and the major source of estrogenic stimulation has ceased. Compared to women aged 25–29 years, the relative risk of endometriosis is 2.1 for women aged 30–34 years, 4.5 during the ages 35–39 years, and finally 6.1 for ages 40–44 years.
Pathogenesis of Endometriosis
Many theories have been proffered to explain the histogenesis of endometriosis. These are not necessarily mutually exclusive. The most widely discussed hypotheses include transplantation of endometrial fragments to ectopic sites, and metaplasia of the multipotential celomic peritoneum. The vast clinical experience and experimental studies in non-human primates favor an etiology that includes transplantation of exfoliated endometrial cells. Clonality studies of endometriotic cysts have shown that the epithelial component is monoclonal.23,24 These results suggest that endometriotic cysts are outgrowths from a single cell of origin, consistent either with transformation of a single progenitor cell, or rare cells within transplanted tissue fragments capable of developing into endometriosis.
Transplantation
The most easily understood, scientifically supported, and widely accepted mechanism is that, at menstruation, some of the menstrual products flow in a retrograde fashion through the lumen of the fallopian tubes into the pelvic peritoneal cavity.25 The material drops to the pelvic floor and implants, in time regenerating into recognizable endometrium. This pathophysiology explains the most common sites of endometriosis. Abundant evidence indicates that menstrual retrograde flow does happen and, indeed, is a common phenomenon. Over 90% of women have blood in their pelvis at the time of menstruation, as identified by fluid examined at laparoscopy (Figure 22.1). Retrograde flow is, in itself, insufficient to cause endometriosis as only a small proportion will develop disease. Host inflammatory or immunologic factors may modify the outcome. Alternatively, sporadically acquired epigenetic or genetic changes within displaced endometrial tissues may alter the likelihood of successful implantation or persistence. Altered methylation of the SF1 gene is one such possibility.26
The frequency with which endometriotic implants are found in the pelvic cavity both usually and under special conditions supports an origin from transplanted menstrual products. Studies have shown that the most frequent sites are where the menstrual products flow from the fimbrial ends of the tubes.27 The uterine position is also of importance.28 It is found significantly more commonly anteriorly in patients with severely anteflexed uteri. In contrast, when the uterus is retroflexed, anterior disease is uncommon. The more frequent finding of endometriosis in the left rather than the right ovary suggests that the endometrial retrograde flow is entrapped on entry by a shield formed in part by the sigmoid colon.
Further proof that menstrual material is viable has been obtained by collecting samples of menstrual products and injecting them subcutaneously into the anterior abdominal wall of animals. Excisional biopsy of the injection sites after several weeks showed lesions that resembled endometriosis in some subjects.7
Animal studies also support that menstrual products have the potential to implant and grow on a peritoneal surface. In baboons, a species with a high rate of spontaneous endometriosis, blood-stained peritoneal fluid was 10-fold more frequent (62% vs 6%) during menses than during non-menstrual phases.29 Retrograde menstruation was also observed more frequently in animals that had spontaneously developed endometriosis than in animals that were free of disease and had a normal pelvis. Finally, the experimental intrapelvic injection of menstrual endometrium led to the development of endometriosis much more frequently than if normal secretory endometrium was injected.30
Several other routes of transplantation, i.e., vascular spread, lymphatic spread, and direct implantation, have been observed. All help explain the occurrence of disease at distant or unusual sites. Endometriosis in a location such as within the parenchyma of the lung is usually accepted as vascular spread. Fragments of endometrium may be seen in lymphatics in about 5% of women with endometriosis,31,32 and occasionally in lymph nodes,33 a phenomenon easiest explained by lymphatic spread. Finally, endometriosis in operative scars and in the cervix after cone biopsy or loop excision is easy to understand, as the endometrial tissue reaches the site by spillage at the time of operation and direct implantation.
The occurrence of endometriosis in young girls has been used as an argument that endometriosis arises by metaplasia, although, when occurring in young women, it is usually perimenarchal and accompanied by outflow obstruction caused by structural anomalies.31
Metaplastic Theory
The metaplastic theory, for which there are scant data, proposes that endometriosis arises in the pelvis and elsewhere by endometrial metaplasia of the peritoneal serosa or serosa-like structures. The concept of the so-called ‘secondary müllerian system’34 attributes to the pelvic peritoneum the ability to differentiate, if appropriately stimulated, into any of the recognized types of müllerian epithelium.
One argument that proponents for both sides of the controversy have made concerns case reports of development of endometriosis in women who congenitally lack a uterus or do not menstruate.35 One case, initially published as an example of endometriosis with an absent uterus, was later reported after new operative findings disclosed a functioning endometrium in a right rudimentary horn and retrograde menstruation through a fallopian tube with hematosalpinx.36 In another instance, a 24-year-old woman with mosaic Turner syndrome was found to have endometrioma arising from the uterine serosa. However, as she had been receiving cyclic hormone-replacement therapy (HRT) for 5 years after laparoscopic gonadectomy and was having cyclic menstrual flow, it is likely that the endometriosis arose from retrograde menstruation.37
Intrinsic Abnormalities of Endometriotic Tissue
There are qualitative and quantitative differences between eutopic and ectopic endometrial tissues, which explain some aspects of lesion persistence and pain. The reader is referred to any of several excellent recent reviews for further details,15,38 summarized in Figure 22.2 and the following sections.
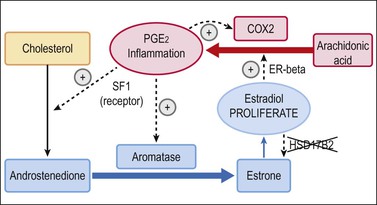
Figure 22.2 Unbroken cycle of inflammation and proliferation in endometriosis. The presence of aromatase (absent in eutopic endometrium) and abnormally high levels of COX2 in endometriotic tissues creates unchecked positive feedback loops for inflammation (red) and proliferation (blue). The cycle is augmented by additional changes not seen in native endometrium, including presence of SF1 and deficiency of HSD17B2.15
Local Feedback between Estrogen Production and Inflammation
Aberrant expression of COX2 in endometriosis, which is normally present at only low levels in eutopic endometrium, leads to overproduction of the inflammatory mediator prostaglandin, PGE2. In addition to the cascade of secondary inflammatory effects normally mediated by PGE2, endometriotic tissues are defective in having a ‘short circuit’ between the normally independent inflammatory (Figure 22.2, red elements) and endocrine synthetic (Figure 22.2, blue elements) pathways. The inflammatory response provides positive feedback to the steroidogenic pathway of endometriotic tissues by abnormal activity of two genes: SF1, and aromatase. SF1 is a nuclear receptor that positively regulates synthesis of androstenedione through a series of intermediaries. Epigenetic modification of the SF1 promoter, by hypomethylation, is one possible mechanism of its overexpression in endometriotic tissues.26 Androstenedione is not normally converted to estrogen in the endometrium, but, as endometriosis has high levels of aromatase, it is converted efficiently to estrone. Estrone has only low-level bioactivity in promoting proliferation of endometrial tissue, but can in turn be converted to the much more potent estradiol. Equilibrium between estrone and estradiol levels is disrupted in favor of the latter by specific inactivation of HSD17B2 in endometriosis. The resultant estradiol, produced locally as above, is locally proliferative and closes the connection between hormonal and inflammatory pathways through its positive feedback on COX2 activity.
Altered Stromal–Epithelial Interaction
The response of ectopic endometrial tissues to circulating steroid sex hormones is perturbed in a manner that confounds normal paracrine inhibition of glandular proliferation by stromal cells.38 Normal endometrial stroma typically expresses progesterone receptors, enabling stromal cells to respond to circulating progesterone by paracrine secretion of retinoic acid. Upon exposure to locally secreted retinoic acid, the glandular cells convert estradiol to less active estrone, thereby mitigating the glandular mitogenic effects of estradiol. This paracrine mechanism of suppressing gland proliferation is compromised in endometriosis tissues, as the stromal (but not glandular) cells have reduced progesterone receptors.
Etiologic Factors in Endometriosis
Genetic Factors
Heritable factors are important in the development of endometriosis.39–41 In an early study of women with histologically proven endometriosis, a surprisingly large proportion of their female relatives were similarly affected (6% of sisters, 8% of mothers, and 7% of first-degree female cousins). In contrast, only 1.0% of their husbands’ sisters and 0.9% of their husbands’ mothers were affected. Furthermore, those women with endometriosis whose first-degree relatives were affected were more likely to have a severe form of the disease than those women whose first-degree relatives were not affected. In another study comparing 515 cases with 149 control cases (women without endometriosis determined by laparoscopy performed during sterilization), endometriosis was found in 3.9% of mothers of cases but in only 0.7% of mothers of controls. It was also found in 4.8% of sisters of cases, but only 0.6% of sisters of controls. The relative risk of endometriosis in a first-degree relative was 7.2. The manifestations of endometriosis were far more severe in women with a positive family history than in those without (26% vs 12%).42 Recent pilot surveys also suggest that there are high concordance rates for the presence of endometriosis in monozygotic but not dizygotic twins.43,44 Large international collaborative projects are currently being undertaken to identify the multifactorial mode of inheritance.45
Endometriosis risk has been reported to be increased in women who carry specific pleomorphisms of vascular endothelial growth factor (VEGF), a gene that promotes angiogenesis.46 A possible mechanism is enhanced VEGF biologic activity of the VEGF +936TC gene polymorphism, thereby facilitating implantation and growth of displaced endometrial tissues. The magnitude of increased risk is small, of the order of 1.18-fold.
Congenital Anatomic Abnormalities
Endometriosis in young girls does not occur much before the age of 11 years,47 and when occurring in young women shortly after the onset of their menarche, is found associated with müllerian anomalies causing outflow tract obstruction.31 On average, the elapsed time between the onset of menarche and the development of symptoms that require surgical intervention is 3 years if the woman has müllerian anomalies with outflow tract obstruction; it is nearly 7 years if outflow tract obstruction is present but the pelvic anatomy is normal.
Systemic Hormonal Factors
Multiple lines of evidence support the importance of ovarian sex hormones in the genesis of endometriosis. Endometriosis is more common in women and primates where there are prolonged periods of unopposed estrogen exposure or in obese women who have higher levels of endogenous estrogen.7 Conversely, it is much rarer in women who have decreased estrogen production during reproductive life. It often disappears after menopause or, when found on histologic examination, appears in an atrophic state. It may also regress with medical suppression, but reappears once ovarian activity resumes.48 The relative contributions of circulating estrogen and progesterone are difficult to study independently, as they dynamically coexist in most women.
There are several emerging lines of evidence supporting a role for systemic and local progesterone effects in the development of endometriosis. Selective progesterone receptor modulators, which block the progesterone response through competitive interaction with the progesterone receptor, may be effective in treating endometriosis.49 Larger prospective clinical trials of efficacy and demonstration of endometrial safety are needed before suitability for routine clinical use can be determined. As mentioned earlier, eutopic and ectopic endometrial tissues differ in their ability to respond to circulating progesterone, as the stroma of the former but not the latter contains progesterone receptors.
Peritoneal Environment
The hormonal composition of the peritoneal environment is enriched by proximity of the ovaries, and local release of follicular fluid. Steroid hormone concentrations are exceedingly high in ovarian follicles, measuring 1000-fold higher than in plasma.50 During the follicular phase, the concentration of estradiol in the peritoneal fluid increases progressively, rising after ovulation to a maximum of 40,000 pg/mL, which is a level 100 times that in the plasma. Progesterone levels both mirror and are higher than plasma levels. During the follicular phase, the levels in the peritoneal fluid are low (5–10 ng/mL), but jump abruptly after ovulation to 2000 ng/mL, decreasing slowly thereafter.
Endometriosis may be linked to abnormal immune function, a possible explanation for why only some women develop the disease even though retrograde menstruation is so common.51 In addition to the hormonal milieu established by the ovary, peritoneal fluid contains multiple types of free-floating cells, including macrophages, mesothelial cells, leukocytes, lymphocytes, eosinophils, and mast cells. Macrophages are estimated to account for over 80% of the cells in normal women.52 In women with endometriosis, there is a pronounced increase in the number of total leukocytes, including macrophages, helper T-lymphocytes, and natural killer cells, supporting the suggestion that active immunologic processes are occurring. Whether the changes observed are cause or effect remains unknown.
The differences that exist between superficial implants and those deeper (indicated in current classifications) may reflect the different local microenvironments to which each area of endometriosis is exposed. Peritoneal fluid factors would be expected to have a greater effect on superficial implants, whereas blood and ovarian hormonal factors regulate deep-seated endometriosis and cystic ovarian endometriosis.50 Endometriotic tissue that has penetrated at least 5 mm beneath the peritoneal surface, and is therefore deep, correlates with more aggressive behavior as reflected by pelvic pain and infertility.53
Angiogenesis
Under the transplantation theory, the retrograde endometrial flow must attach to and implant within the peritoneal cavity, and then establish and maintain an adequate blood supply. Growth of a new vascular bed is therefore critical if the endometriosis is to develop. A wide array of factors contribute to angiogenesis, and it has been suggested that basal activity of several polymorphic variants may alter the risk for endometriosis.46,54
Some evidence suggests that a key to angiogenic activity may lie in the contents of the peritoneal cavity rather than in the endometriotic explant itself.55 Potent angiogenic growth factors are increased in the peritoneal fluid in patients with this disease. The activated peritoneal fluid macrophages and infiltrating macrophages are a rich source of this angiogenic growth factor.
Morphologic Features of Endometriosis
In the most elemental terms, endometriosis can be diagnosed by finding both endometrial glands and stroma in the operative specimen. The appearances, however, can be protean and affected by topography, age of the lesion, and age of the patient. The laparoscopic appearances of the lesions are depicted in Figures 22.3–22.7.
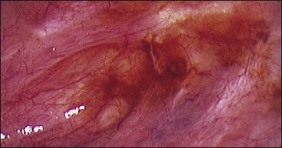
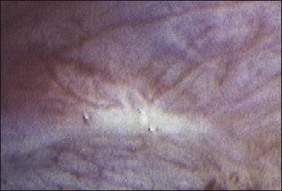
Figure 22.3 Yellow-red stain of endometriosis. This is the earliest manifestation of endometriosis seen laparoscopically.
Gross Features
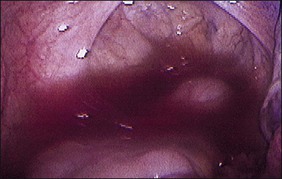
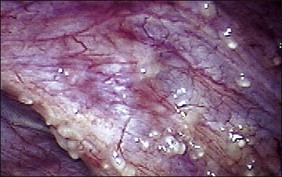
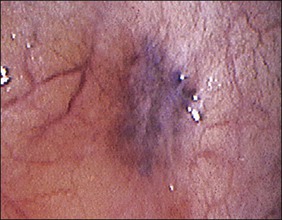
The ages of the endometriotic deposits affect their gross appearances. The various colors reflect the deposit’s functional states. Yellow-red surface stains, which reflect breakdown of blood products on the involved surfaces, often herald the presence of the earliest detectable lesions (Figure 22.3). Occasionally, early lesions will show vesicle formations (Figure 22.4), a state before the endometriotic foci begin to cycle and undergo tissue and blood breakdown. The red lesions also reflect an early form of the disease when the endometriosis is actively growing (Figure 22.5).56 These red lesions evolve into the black (advanced) lesions in which some degree of bleeding has resolved (Figure 22.6). This is the most common form seen by the pathologist in operative specimens. Some lesions may be brown to slightly yellow-brown, which indicates the presence of hemosiderin. These lesions are also called ‘café-au-lait spots.’ The oldest (white) lesions, have fibrosis and scarring and often the color reflects the advanced degree of healing (Figure 22.7). Strangely, it is the white lesion where endometriosis is most easily confirmed histologically.57 As lesions begin at different times, the various foci may differ from site to site. Seldom are endometriotic lesions solitary.
The changes seen laparoscopically are also seen macroscopically in the surgical specimen. In general, the first grossly recognizable lesions are blister-like blebs, some 2–3 mm in diameter, on the surface of the target organs. They are red and represent highly vascularized implants.58 The tiny red lesions are the exclusive form of endometriosis found in 20% of adolescents with this disease. They have yet to show signs of repeated bleeding and scarring (fibrosis). Some investigators59 believe that many of the red lesions should not be considered as endometriosis, assuming that virtually all women have retrograde menstruation and therefore some red lesions.
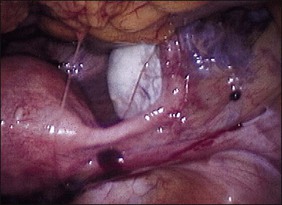
As the lesions advance in age, they enlarge, reaching a size of 3 mm to sometimes over 1 cm, sometimes singly or as a collection. As they go through repeated cycles, they become pigmented and blacker, largely from intraluminal debris, old blood, hemorrhagic stroma, hemosiderin-laden macrophages, and even some scarring. These lesions may be raised, bluish-red to bluish-black, and may resemble mulberries or blueberries (Figure 22.8). If they are more extensively fibrotic and scarred, they may also appear puckered (‘powder burn’) (Figure 22.9). Lesions that heal have undergone extensive fibrosis and scarring and are white. They have minimal stroma and are poorly vascularized. Black-and-white scarred lesions predominate in later years.
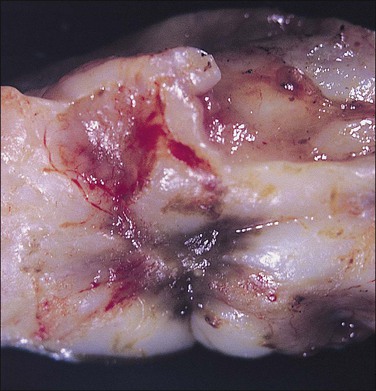
Figure 22.9 Scarred (‘powder burn’) lesion of endometriosis. The consequent extensive fibrosis has resulted in a puckered surface.
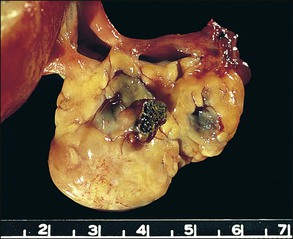
The disease site affects whether the endometriosis is serosal (including subserosal/subperitoneal) or whether it can be found deep within an organ (e.g., ovary). Implants of endometriosis grow poorly on the surface of the peritoneal mesothelium.60 Rather, mesothelium quickly grows over the implant, encapsulating it as if it were a foreign body; such nodules appear to be located subperitoneally. In the ovary, these nodules enlarge during cyclical ‘menstruation,’ first appearing as small indentations into the cortex, and with time invaginating substantially into the ovarian substance. On cross section, these foci of developing endometriomas show the wall and base to actually consist of the ovarian cortex/serosa, the so-called ‘inverted cortex’ (Figure 22.10). Sometimes, the pearl-white appearance of the ovarian cortex is still recognizable. The tissue covering the endometrioma, i.e., the peritoneal cover, is an operculum consisting of fibrous tissue and mesothelium.
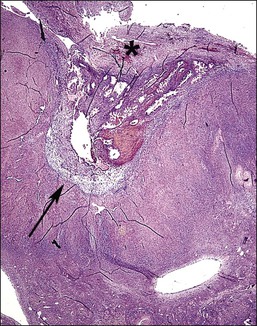
Figure 22.10 Inverted cortex of ovarian endometrioma. The inverted ovarian cortex lies at the base (arrow) of the endometrioma. A fibrous operculum (*) covers the upper surface of the endometrioma.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree