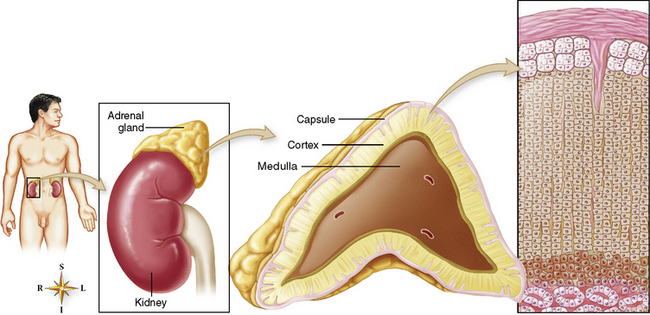
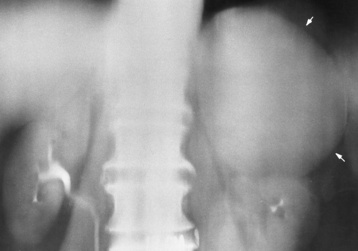
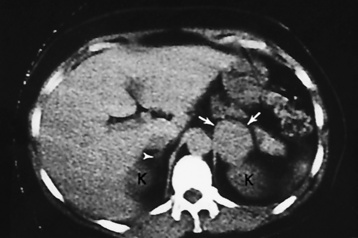
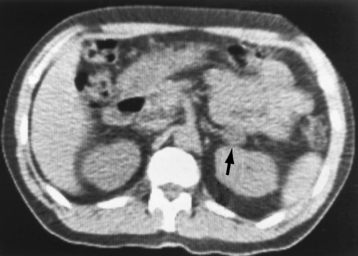
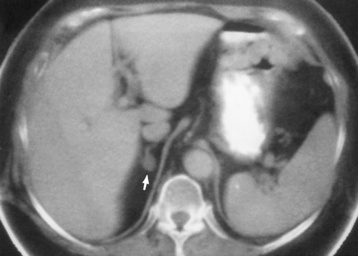
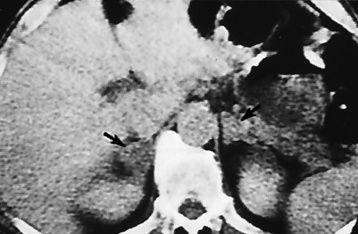
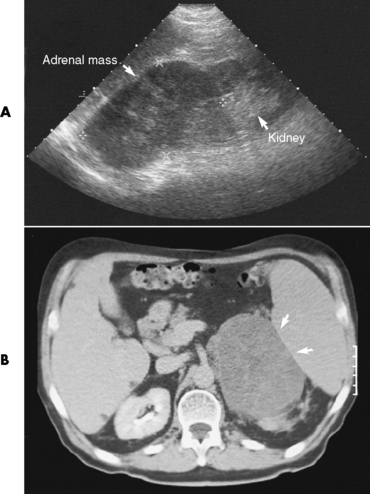
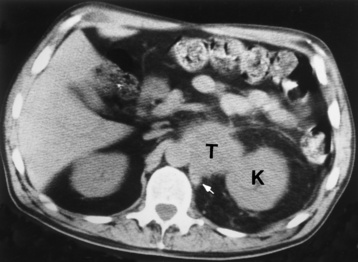
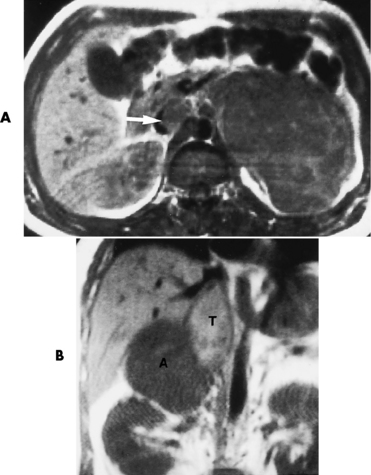
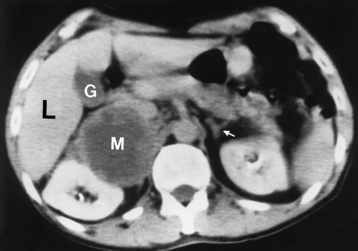
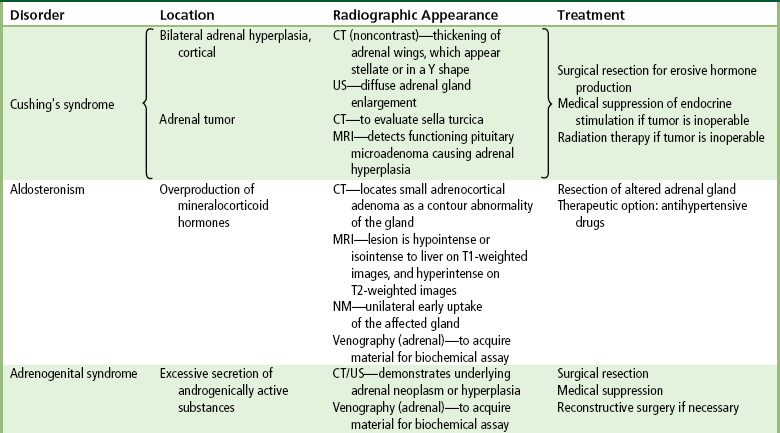
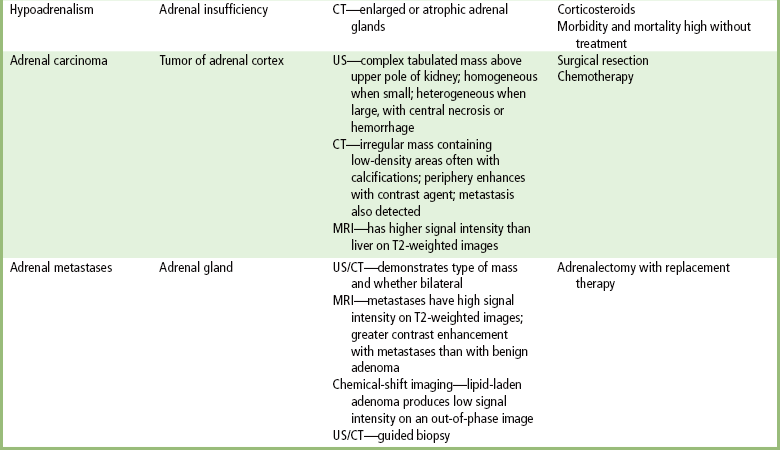
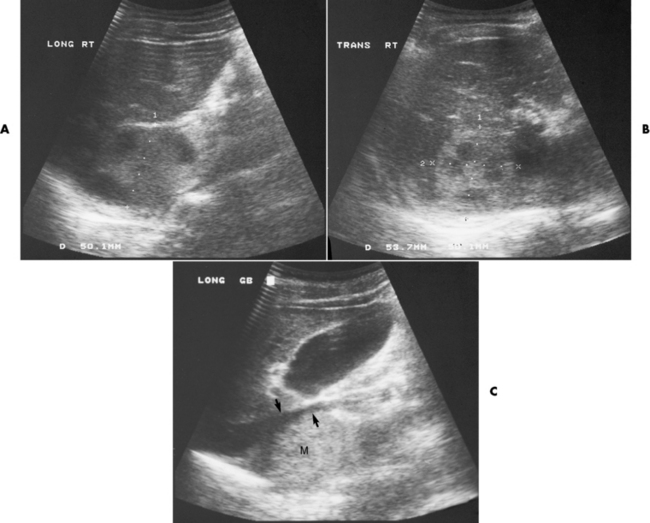
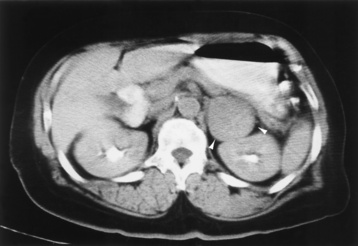
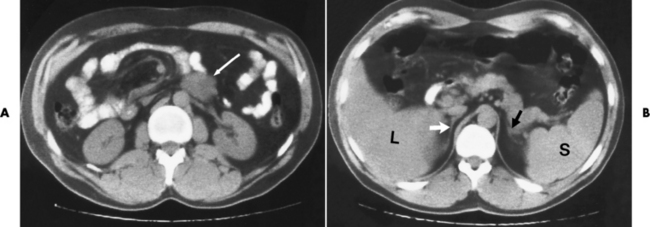
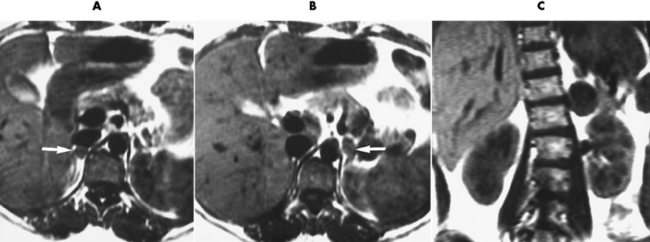
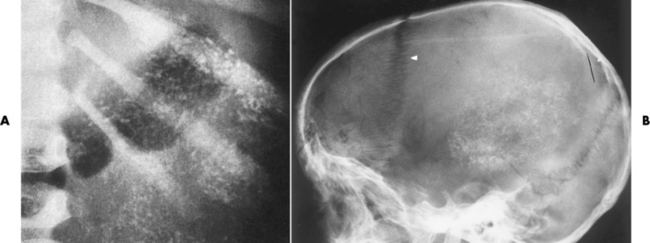
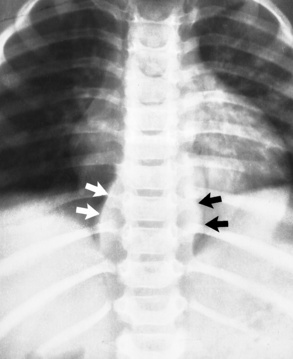
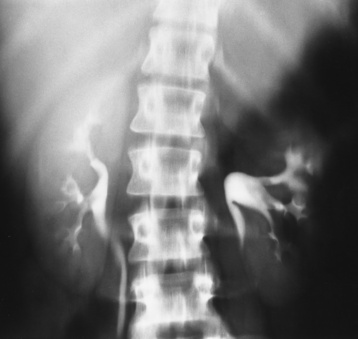
Chapter 10 After reading this chapter, the reader will be able to: 1 Define and describe all bold-faced terms in this chapter 2 Describe the physiology of the endocrine system 3 Identify anatomic structures on both diagrams and images of the endocrine system 4 Differentiate the various pathologic conditions affecting the endocrine system and their radiographic manifestations The major endocrine glands are the pituitary, adrenal, thyroid, and parathyroid glands. Inadequate (hypoactive) or excess (hyperactive) production of hormones from these endocrine glands can give rise to a wide variety of clinical symptoms and radiographic abnormalities. Physiology of the Adrenal Glands Each of the adrenal glands, which are situated at the top of each kidney, consists of an outer cortex and an inner medulla (Figure 10-1). The adrenal cortex secretes several different types of steroid hormones, which can be divided into three general groups. The mineralocorticoids (primarily aldosterone) regulate salt and water balance by controlling sodium retention and potassium excretion by the kidneys. The production of aldosterone is regulated primarily by the secretion of renin from specialized cells (the juxtaglomerular apparatus) in the kidney. Reduced blood volume (as in hemorrhage) causes low blood pressure, which is detected by the juxtaglomerular apparatus and eventually results in increased aldosterone secretion from the adrenal cortex. Glucocorticoids (especially cortisone) regulate carbohydrate metabolism and are under the regulation of adrenocorticotropic hormone (ACTH) from the anterior pituitary gland. Cortisone also depresses the inflammatory response to almost all forms of injury, thus leading to its use in the treatment of trauma, rheumatoid arthritis, bursitis, and asthma, and as an immunosuppressive agent to help limit rejection after organ transplantation. Androgens are sex hormones that tend to masculinize the body, to retain amino acids, and to enhance protein synthesis. It is these hormones that are used both illegally and unwisely by athletes in an attempt to increase body strength. Radiographic Appearance: Generalized enlargement of the adrenal glands is best demonstrated by computed tomography (CT), which shows thickening of the wings of the adrenal gland, which appear to have a stellate or Y-shaped configuration in cross section. Ultrasound can also show diffuse adrenal gland enlargement. Benign and malignant tumors of the adrenal cortex are less common causes of Cushing’s syndrome than is nontumorous adrenal hyperfunction. As a general rule, the larger the adrenocortical tumor and the more abrupt the onset of clinical symptoms and signs, the more likely the tumor is to be malignant (Figure 10-2). However, the differentiation between adenoma and carcinoma may be impossible at the time of histologic examination, and the nature of the tumor may have to be defined by the clinical course alone. Both CT and ultrasound can demonstrate an adrenal tumor (Figure 10-3), but CT is often more valuable because the abundance of retroperitoneal fat may prevent an optimal ultrasound examination. Adrenal venography has been widely used to demonstrate adrenal masses, and it also permits the aspiration of blood samples for assessment of the level of adrenal hormones. Treatment: Treatment for Cushing’s syndrome depends on the cause of the excess production of glucocorticoid hormones. Surgical resection is the treatment of choice to eliminate the excess hormone production. Medical suppression of abnormal endocrine stimulation and radiation therapy directed to the hyperfunctioning tumor are alternatives if the tumor is inoperable. Radiographic Appearance: The role of diagnostic imaging is to demonstrate the location of adenomas that may otherwise be difficult to detect during exploratory surgery. Noncontrast CT, the most widely used imaging modality, demonstrates the small adrenocortical adenoma as a contour abnormality of the gland (Figure 10-4). With use of CT or MRI, the adrenal gland can be measured quite accurately; the normal adrenal measures 3 to 6 mm thick, 4 to 6 mm long, and 2 to 3 cm wide. However, the scan or image may not demonstrate any abnormal findings. With newer CT scanners, specificity has increased to 75%, and tumors larger than 1 cm can be consistently identified. Adenomas may be isointense or hypointense relative to the liver on T1-weighted MR images. On T2-weighted images, they have slight hyperintensity. Chemical-shift imaging can aid in identifying and characterizing an adrenal mass. In adenomas smaller than 1 cm, nuclear medicine studies using I-131 attached with a cholesterol binder can differentiate a normal gland from hyperplasia on the basis of the time course of radionuclide uptake. Early uptake (less than 5 days) in both adrenal glands indicates hyperplasia, whereas unilateral early uptake implies an adrenal adenoma. Adrenal venography with biochemical assay of a sample of adrenal blood is another important technique for localizing aldosteronomas and determining whether the hyperaldosteronism is primary or secondary. Treatment: Hypertension and other clinical manifestations of aldosteronism can be cured by the resection of an adenoma but are little affected by the removal of both adrenal glands in the patient with bilateral hyperplasia. Medical antihypertensive agents, especially long-acting calcium channel blockers, are available if the adenoma is inoperable. Radiographic Appearance: Most cases of acquired adrenogenital syndrome are caused by adrenocortical tumors, which can be detected by CT (Figure 10-5), ultrasound, or adrenal venography. CT is currently the imaging modality of choice. Treatment: Surgical resection of a hyperfunctioning tumor with replacement therapy allows normal development. Medical suppression of abnormal stimuli prevents the excessive release of ACTH, allowing female features to develop normally. If not treated, a masculinized woman may require reconstructive surgery. Radiographic Appearance: Acute inflammatory disease causes generalized enlargement of the adrenal glands, which can be demonstrated by a variety of imaging techniques (Figure 10-6). MRI can differentiate adrenal masses better than CT, but MRI cannot distinguish a tumor from an inflammatory process. Other radiographic findings occasionally seen in patients with adrenal insufficiency include a small heart and calcification of the cartilage of the ear. Radiographic Appearance: Ultrasound demonstrates the tumor as a complex mass that may be difficult to separate from an upper pole renal tumor (Figure 10-7). CT demonstrates an adrenal carcinoma as a large unilateral mass with an irregular edge that often contains low-density areas resulting from central necrosis or prior hemorrhage (Figure 10-8) and high-density calcifications. On contrast-enhanced CT, the tumor enhancement is irregular and greatest on the periphery. For adrenal examinations, spiral CT with 3- to 5-cm section reconstructions offers the best resolution and may identify tumors 1 cm or smaller. Because lymphatic and hepatic metastases are common at the time of clinical presentation, CT scans at multiple abdominal levels are necessary to define the extent of the primary tumor and to detect metastases before surgical resection is attempted. Extension of the tumor into the renal vein and inferior vena cava can also be detected by CT, especially after the injection of intravenous contrast material, or by MRI (Figure 10-9). On MR images, the higher signal intensity of the tumor in comparison with the liver on T2-weighted images (lower signal intensity on T1-weighted images) may distinguish adrenal carcinoma from nonfunctioning adenomas and pheochromocytomas. Radiographic Appearance: Metastatic enlargement of an adrenal gland can cause downward displacement of the kidney with flattening of the upper pole. Ultrasound and CT demonstrate adrenal metastases as solid, soft tissue masses that vary considerably in size and are frequently bilateral (Figure 10-10). However, the ultrasound (hypoechoic lesions) and CT patterns are indistinguishable from those of primary malignancies of the gland. Therefore, when a known primary tumor exists elsewhere, it is usually assumed that an adrenal mass is metastatic. Radiographic Appearance: CT and ultrasound (Figure 10-11) are very useful in the localization of pheochromocytomas. The cross-sectional images not only detail the extent of the adrenal lesion but also define the status of adjacent structures and can demonstrate bilateral or multiple pheochromocytomas, extraadrenal tumors, and metastases. Pheochromocytomas generally appear as round, oval, or pear-shaped masses, often greater than 3 cm, that are slightly less echogenic than liver and kidney parenchyma on ultrasound and have an attenuation value less than these organs on CT (Figure 10-12). Necrosis, hemorrhage, and fluid levels are common findings in larger lesions. Most extraadrenal pheochromocytomas arise in the abdomen (Figure 10-13); a few are found in the chest or neck. The tumor may be located anywhere along the sympathetic nervous system, in the organ of Zuckerkandl, and in chemoreceptor tissues such as the carotid body and the glomus jugulare, in the wall of the urinary bladder, or even in the kidney or ureter. Masses may displace the ureter or kidney or may appear as filling defects in the bladder. MRI can demonstrate the relationship of the tumor to surrounding structures in the coronal plane (Figure 10-14) and has a higher sensitivity than CT. On T2-weighted spin-echo images, the extreme hyperintensity of the tumor (because of its water content) makes it stand out from the surrounding structures. When CT and MRI findings are inconclusive, a radionuclide scan using metaiodobenzylguanidine is highly sensitive for localizing ectopic pheochromocytomas, but this agent is not readily available. Radiographic Appearance: Calcification is common in neuroblastoma (occurring in about 50% of cases), in contrast to the relatively infrequent calcification in Wilms’ tumor, from which neuroblastoma must be differentiated. Calcification in a neuroblastoma has a fine granular or stippled appearance (Figure 10-15A). Occasionally, there may be a single mass of amorphous calcification. Calcification can also develop in metastases of neuroblastoma in the paravertebral lymph nodes and the liver. Intravenous urography usually demonstrates downward and lateral renal displacement by the tumor mass (Figure 10-16). Neuroblastoma tends to cause the entire kidney and its collecting system to be displaced as a unit, unlike Wilms’ tumor, which has an intrarenal origin and thus tends to distort and widen the pelvicalyceal system. Figure 10-16 Neuroblastoma. Nephrotomogram demonstrates downward and lateral displacement of the upper pole of the left kidney. Because of its nonionizing character, ultrasound is a superb modality for evaluating abdominal masses in children. A neuroblastoma appears as a solid or semisolid mass that is separate from the kidney. It appears as a poorly defined heterogenic mass, unlike the well-defined and relatively homogeneous Wilms’ tumor. A neuroblastoma is often diffusely hyperechogenic, probably because of necrosis, calcification, and hemorrhage. On contrast-enhanced CT, necrosis and hemorrhage cause the tumor to appear heterogeneous and often lobulated. Because CT can demonstrate evidence of tumor spread to lymph nodes and the sympathetic chain, widening of the paravertebral stripe (also seen on plain images) (Figure 10-17), and metastases to the liver and chest, it has become the most commonly used imaging study to diagnose neuroblastomas. This modality is also used to assess the response to treatment. MRI, which like ultrasound does not use ionizing radiation, may offer a safer approach to demonstrating characteristics typical of tumor tissue. Neuroblastomas usually produce a hypointense signal on T1-weighted images and are hyperintense on T2-weighted images. On contrast-enhanced T1-weighted images, hemorrhage appears as a hyperintense signal.
Endocrine System
Physiology of the endocrine system
Adrenal glands
Diseases of the Adrenal Cortex
Aldosteronism
Adrenogenital Syndrome
Hypoadrenalism
Adrenal Carcinoma
Metastases to the Adrenal Gland
Diseases of the Adrenal Medulla
Neuroblastoma
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree