Fig. 20.1.
Lingual thyroid : depression at the site of excision of lingual thyroid.
Microscopic (Fig. 20.2 )
♦
Heterotopic and ectopic thyroid tissue is a histologically normal thyroid tissue, but can manifest pathologic processes that affect the thyroid gland (hyperplasia, neoplasia, inflammation, etc.). Individual follicles may proliferate between adjacent structures (such as muscle) simulating invasion
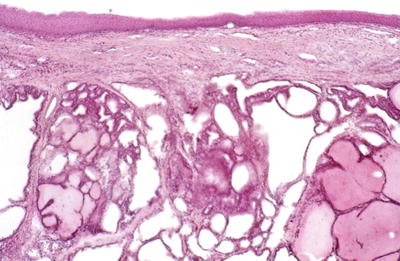
Fig. 20.2.
Lingual thyroid : variable-sized hyperplastic follicles , some lined by cubical cells and featuring occasional papillary infoldings.
Differential Diagnosis
♦
Metastatic thyroid carcinoma
Follicular carcinoma or follicular variant of papillary carcinoma may resemble normal thyroid tissue; thus, careful examination for solid areas, necrosis, mitoses, anaplasia, true invasion, or cytologic features of papillary thyroid carcinoma is needed to rule out a metastasis
Parasitic Nodule
Clinical
♦
A parasitic nodule is a thyroid nodule in the neck that is anatomically separate from the main thyroid gland. The background thyroid gland is often a multinodular goiter. The parasitic nodule probably results from a colloid or hyperplastic nodule located outside of the thyroid gland, which becomes enlarged and migrates laterally in the neck
Macroscopic
♦
Parasitic nodules range in size from 1 to 4 cm and may be connected to the thyroid gland by a thin strand of fibrovascular tissue or may be completely separate, with a blood supply from the surrounding tissues
Microscopic
♦
The histologic appearance is similar to normal thyroid tissue with hyperplastic or colloid-filled follicles
Differential Diagnosis
♦
Metastatic thyroid carcinoma
The presence of many lymphoid cells in a patient with Hashimoto thyroiditis and a parasitic or sequestered nodule may lead to a mistaken diagnosis of metastatic carcinoma to a lymph node. Thus, careful determination of the exact location of the tissue is important as well as the histologic features
Thyroglossal Duct Cyst
Clinical
♦
Thyroglossal duct cyst is a cystic dilation of thyroglossal duct remnant, found along the course of thyroglossal duct (from the posterior tongue down along the anterior midline to below cricoid cartilage). The duct may run anterior to, within, or posterior to the hyoid bone
♦
Thyroglossal duct cysts are usually present in childhood with a midline nodule that may be tender and can be associated with the sinus tract. Fistulas may develop secondary to infection and may open into the pharynx or skin
♦
Heterotopic thyroid tissue associated with the cyst may give rise to thyroid malignancies (papillary carcinoma)
Macroscopic
♦
Thyroglossal duct cyst is filled with mucin and measures up to 3 cm in diameter. Normal thyroid tissue may be seen in the wall of the cyst
♦
Thyroglossal duct cysts are usually lined by pseudostratified ciliated or squamous epithelium. The epithelial lining may not be identified if inflammation is prominent. The surrounding stroma shows thyroid follicles and inflammation
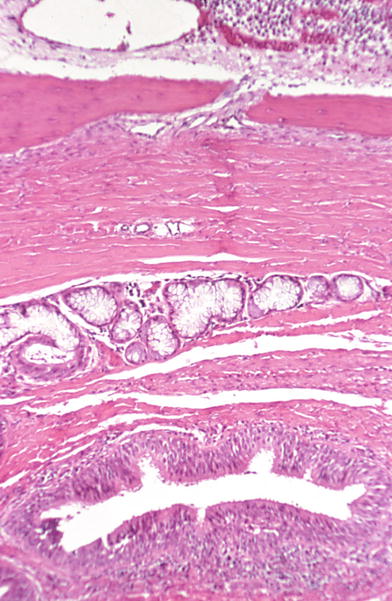
Fig. 20.3.
Thyroglossal duct: duct lined by columnar epithelium with lymphocytic infiltrate . Mucous glands and striated muscle separate the duct from the hyoid bone.
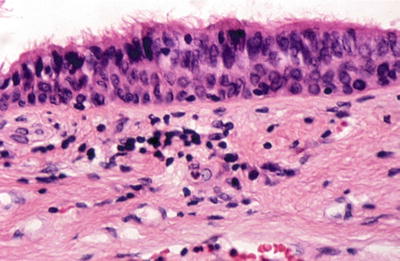
Fig. 20.4.
Thyroglossal duct: pseudostratified ciliated columnar epithelium line duct.
Differential Diagnosis
♦
Heterotopic thyroid
Heterotopic thyroid shows only thyroid tissue, with no cyst or duct lining
♦
Branchial cleft cyst
Located in the anterolateral neck, branchial cleft cysts lack the classic midline location of thyroglossal duct cysts. Branchial cleft cysts are lined by mixed squamous and respiratory epithelium and are associated with lymphoid follicles, but no thyroid tissue is seen
♦
Thyroid neoplasm with cystic degeneration
Follicular adenoma, adenomatous goiter, or carcinoma may show cystic change. Thus, careful examination of the cyst wall and surrounding tissue is important
Acute Thyroiditis
Clinical
♦
Acute thyroiditis is usually caused by bacteria, although fungi and viruses have also been implicated. Usually, the thyroid is secondarily involved by a systemic infection or local infection in the neck
♦
Patients often present with chills, fever, and malaise and an enlarged and painful thyroid. Gram-positive organisms such as Staphylococcus aureus, Streptococcus pyogenes, Streptococcus epidermidis, and Streptococcus pneumoniae are most common, but Gram-negative bacilli can also be isolated
Macroscopic
♦
The thyroid may be enlarged, with areas of necrosis and abscesses
Microscopic
♦
Acute thyroiditis is characterized by acute inflammation of the thyroid gland with neutrophils and microabscess formation as well as foci of necrosis. Special stains for bacteria and fungal organisms can help identify the organism
Differential Diagnosis
♦
Other types of thyroiditis (granulomatous or lymphocytic)
In other types of thyroiditis, predominant inflammatory cells are either histiocytes or lymphocytes (neutrophils, if present, usually represent minority) and are not associated with other acute infections or trauma
♦
Trauma and ischemic necrosis
Although trauma and ischemic necrosis may present with pain, histologically these entities lack the marked acute inflammation and abscess formation seen in acute infectious thyroiditis
Granulomatous Thyroiditis
Clinical
♦
Granulomatous inflammation occurs in the thyroid in a variety of conditions including infection, sarcoidosis, foreign body reaction, and reaction to hemorrhage, among others
♦
Granulomatous thyroiditis includes nonsuppurative thyroiditis, subacute thyroiditis, and De Quervain thyroiditis
♦
Women are more commonly affected than men
♦
Granulomatous thyroiditis is usually associated with pain and systemic symptoms such as fever, malaise, and weakness. The clinical course of granulomatous thyroiditis has been divided into hyperthyroid phase, hypothyroid phase, and recovery phase
♦
The etiology is unknown, but some cases of granulomatous thyroiditis are thought to be caused by a viral infection, either systemic or an infection of the thyroid itself. Many patients have recently had an upper respiratory infection, and epidemiology studies have shown association with numerous viruses (mumps, Coxsackie, adenovirus, influenza, Epstein Barr, measles, and mononucleosis, among others)
♦
The overwhelming majority of patients recover in a few months. Mild cases are treated with aspirin, while prednisone is used in severe cases
Macroscopic (Fig. 20.5)
♦
The thyroid is enlarged (usually asymmetrical) up to twice the normal size. The cut surface shows areas of irregular firm tan tissue among more normal-appearing thyroid tissue
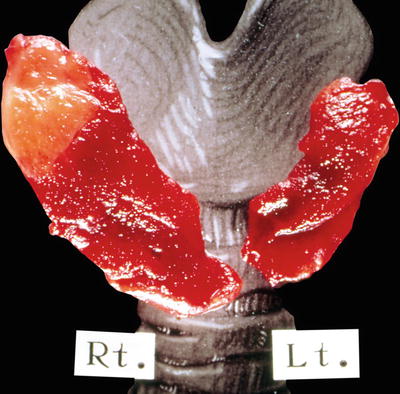
Fig. 20.5.
Granulomatous thyroiditis: localized involvement of the right upper lobe by a demarcated tan-yellow inflammatory.
Microscopic (Fig. 20.6)
♦
Granulomatous thyroiditis is characterized by patchy uneven involvement of the thyroid by neutrophils and microabscesses early to mixed lymphohistiocytic inflammation and vague, noncaseating granulomata with foreign body-type giant cells centered on and destroying follicles and engulfing free colloid. Patchy areas of fibrosis and regenerating follicles may be seen later in the disease
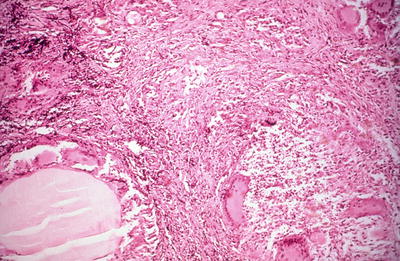
Fig. 20.6.
Granulomatous thyroiditis : normal thyroid parenchyma has largely been replaced by fibrosis and an inflammatory mass, including multinucleated giant cells. Thyroid follicles are in various stages of destruction.
Differential Diagnosis
♦
Autoimmune thyroiditis
Autoimmune thyroiditis shows significantly less inflammation, which is predominantly lymphocytic with germinal centers. There is no gland destruction; rather, follicles show atrophy with Hürthle cell change
♦
Acute thyroiditis
Acute thyroiditis is usually associated with infections (bacterial or fungal) or local trauma. Inflammation is predominantly neutrophilic rather than granulomatous
♦
Tuberculosis or mycosis
Tuberculosis or fungal infections of the thyroid are extremely rare, generally occurring in patients with disseminated systemic involvement. Histologically, the thyroid shows necrotizing granulomas
♦
Sarcoidosis
Sarcoidosis involving the thyroid occurs in patients with systemic sarcoidosis. The granulomas are interstitial and do not result in follicular destruction
♦
Palpation thyroiditis
Palpation thyroiditis is an incidental finding as a result of vigorous palpation of or minor trauma to the thyroid. Histologically, patchy, multifocal mixed lymphocytic and histiocytic foci of inflammation are identified involving isolated and widely distant follicles, in an otherwise histologically normal gland
Riedel Fibrous Thyroiditis
Clinical
♦
Riedel fibrous thyroiditis is a rare, idiopathic inflammatory process manifested by extensive fibrosis of the thyroid gland, extending beyond the gland into the surrounding tissue and usually involving the cervical musculature
♦
Fibrous thyroiditis may be related to fibrosing mediastinitis and retroperitoneal fibrosis (idiopathic fibrosclerosis)
♦
Patients tend to be adult or elderly (average age = 50 years), with slight female predilection. Patients present with painless, poorly defined thyroid enlargement that is often firm to rock hard
Macroscopic (Fig. 20.7)
♦
The thyroid gland is enlarged and hard with white fibrous tissue replacing parenchyma and extending into surrounding tissue, obliterating normal anatomic boundaries and extending into adjacent muscles, nerves, and vessels, making excision difficult or impossible
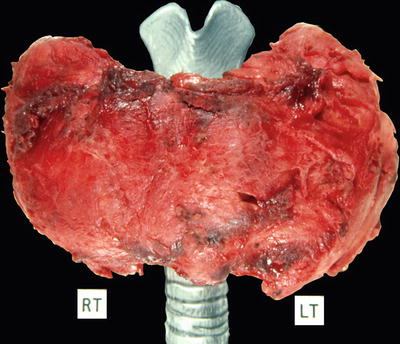
Fig. 20.7.
Riedel fibro us thyroiditis: ragged external aspect of 230 g thyroid gland. Portion of the strap muscles are inextricably attached to the left lobe.
Microscopic (Fig. 20.8)
♦
The thyroid shows extensive fibrosis, infiltrating beyond the thyroid into the muscle and other tissue, with keloid-like hyalinized collagen. Areas of more normal uninvolved thyroid are seen. There is a patchy lymphoid and plasmacytic infiltrate
♦
Involved vessels may show mural inflammation and occlusive phlebitis
♦
Giant cells are not prominent, which helps to differentiate Riedel thyroiditis from granulomatous thyroiditis, although granulomatous thyroiditis lacks that extensive fibrosis seen in Riedel thyroiditis
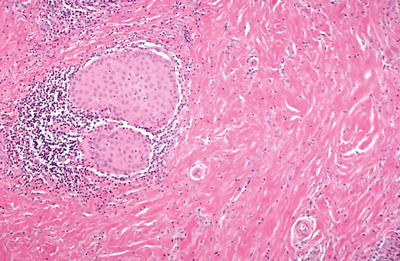
Fig. 20.8.
Microscopic Riedel fibrous thyroiditis.
Differential Diagnosis
♦
Fibrosing Hashimoto thyroiditis
Patients with the fibrous variant of Hashimoto thyroiditis are hypothyroid (although Riedel thyroiditis can also be associated with hypothyroidism), have high antibody titers, and lack pressure symptoms. The thyroid gland in fibrosing Hashimoto thyroiditis is also involved by fibrosis, but the capsule remains intact and may be separated from adjacent structures. Microscopically, fibrosis appears more typical in fibrosing Hashimoto thyroiditis, rather than the keloid-like collagen seen in Riedel fibrous thyroiditis. Occlusive phlebitis and vasculitis may be seen in Riedel thyroiditis, but not in the fibrous variant of Hashimoto thyroiditis
♦
Granulomatous thyroiditis
Giant cells are not prominent in Riedel thyroiditis, which helps to differentiate Riedel thyroiditis from granulomatous thyroiditis. Granulomatous thyroiditis also lacks the extensive fibrosis seen in Riedel thyroiditis
Lymphocytic Thyroiditis
Clinical
♦
Lymphocytic thyroiditis is an autoimmune thyroiditis caused by autoantibodies directed against thyroglobulin and other follicular cell antigens
♦
Lymphocytic thyroiditis is usually seen in children and adults and is more common in women (F:M = 2:1). Patients present with asymptomatic goiter and may have transient hyperthyroidism. The process usually resolves within 1 year
Macroscopic
♦
The thyroid shows mild to moderate enlargement and has a slightly nodular appearance
♦
The thyroid shows scattered interstitial lymphoid follicles with germinal centers. The follicles typically show minimal reaction to inflammation, but there may be some mild atrophy or Hürthle cell change
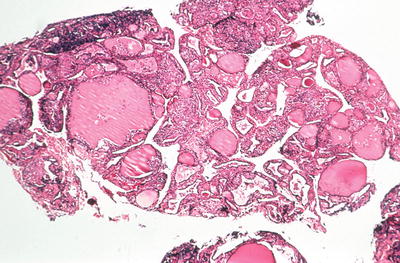
Fig. 20.9.
Lymphocytic thyroiditis (painless thyroiditis): Vim-Silverman needle biopsy of a patient with transient hyperthyroidism. There is a moderately heavy lymphocytic infiltrate involving variable-sized thyroid follicles.
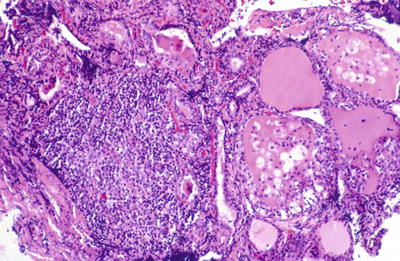
Fig. 20.10.
Lymphocytic thyroiditis (painless thyroiditis): focal heavy lymphocytic infiltrate with follicles showing degenerative changes characterized by swelling of lining follicular cells and inflammatory infiltration.
Differential Diagnosis
♦
Hashimoto thyroiditis
Hashimoto thyroiditis has larger, more numerous lymphoid follicles and scattered plasma cells and histiocytes than lymphocytic thyroiditis. Hürthle cell change is present in Hashimoto thyroiditis
♦
Graves disease
Graves disease is associated with hyperthyroidism. The thyroid shows diffuse follicular hyperplasia often with a prominent papillary architecture
Hashimoto Thyroiditis
Clinical
♦
Hashimoto thyroiditis is an autoimmune thyroiditis characterized by a goiter and circulating antithyroglobulin and antithyroid peroxidase (antimicrosomal) antibodies
♦
Classically, Hashimoto thyroiditis occurs in young to middle-aged females (30–50 years old; F:M = 10:1). Hashimoto thyroiditis is the most common thyroid disease in children and adolescents. Different HLA haplotypes have been associated with Hashimoto disease. Hashimoto thyroiditis is associated with other autoimmune diseases. Hashimoto thyroiditis may be associated with autoimmune disease of adrenals (Addison disease), pancreas (diabetes), or stomach (pernicious anemia). Individuals with Down syndrome have an increased susceptibility to autoimmune thyroid diseases, including Hashimoto thyroiditis
♦
Patients present with diffuse firm goiter, tenderness, and hyperthyroidism. As the disease progresses, thyroid function decreases, eventually resulting in hypothyroidism
♦
There is an increased risk of lymphoma, leukemia, and thyroid carcinoma
Macroscopic
♦
The thyroid gland shows diffuse, firm enlargement. The cut surface may show areas of fibrosis and pale tan color due to the chronic inflammatory infiltrate, unlike the beefy red diffuse appearance of the thyroid in Graves disease. Increasing fibrosis is noted with increasing age
♦
The thyroid shows marked interstitial lymphocytic inflammation with prominent lymphoid follicles with germinal centers. The thyroid follicles are small and atrophic lined by oxyphilic epithelium (Hürthle cells). Interstitial fibrosis may accentuate lobular architecture. Regenerative hyperplasia and squamous metaplasia may be seen
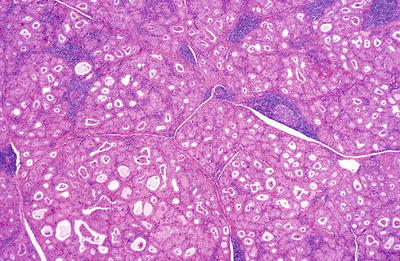
Fig. 20.11.
Hashimoto thyroiditis : the follicles are smaller than normal and lined by oxyphilic epithelium. There is a patchy lymphocytic infiltrate with germinal centers.
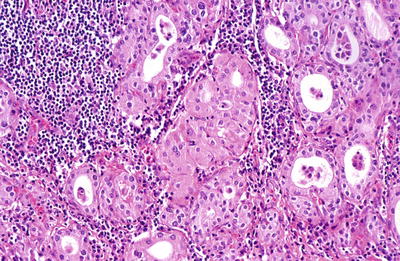
Fig. 20.12.
Hashimoto thyroiditis : oxyphilic cells with granular cytoplasm together with a lymphocytic infiltrate.
Variants
♦
Fibrosing Hashimoto thyroiditis
Approximately 10% of Hashimoto thyroiditis shows prominent fibrosis, which is usually seen in elderly patients. The thyroid shows marked enlargement with some adhesion of gland to surrounding tissue (although surgical planes are readily identifiable). Histologically extensive fibrosis, follicular atrophy with Hürthle cell change, squamous metaplasia, and lymphocytes and plasma cells are seen
This variant may be confused with Riedel thyroiditis as well as carcinoma due to its adherence to adjacent structures. The parenchyma of Hashimoto thyroiditis retains its normal lobular architecture and lacks cytologic features of malignancy
The prominent lymphoplasmacytic infiltrate in Hashimoto thyroiditis can be mistaken for malignant lymphoma. Because thyroid lymphomas usually arise in the setting of Hashimoto thyroiditis, a high index of suspicion is needed when examining thyroid tissues with prominent lymphoid infiltrates
Differential Diagnosis
♦
Lymphocytic thyroiditis
Lymphocytic thyroiditis shows milder inflammation, with scattered lymphoid follicles and little or no reactive changes in thyroid follicles
♦
Graves disease
The follicles in Graves disease appear hyperplastic, rather than atrophic
Postpartum Thyroiditis
♦
Postpartum thyroiditis is an autoimmune disorder, associated with microsomal antibodies, which affects women in the first postpartum year. Postpartum thyroiditis has an early thyrotoxic phase and a longer hypothyroid phase. Histologically, postpartum thyroiditis is shown by focal or diffuse chronic thyroiditis and resembles spontaneous silent thyroiditis. The hypothyroid phase is characterized by follicular disruption and hyperplasia, while focal lymphocytic thyroiditis is seen in the recovery phase
Focal Lymphocytic Thyroiditis
♦
Focal lymphocytic thyroiditis (nonspecific thyroiditis, focal autoimmune thyroiditis) is thought to be an incidental finding, which usually affects elderly, is more common in women than in men, and is relatively common with autopsy studies showing that 20% of thyroids are involved. The thyroid shows dense lymphoid aggregates with or without germinal centers
Graves Disease
Clinical
♦
Graves disease (diffuse hyperplasia) is an autoimmune thyroiditis caused by autoantibodies directed against thyroid-stimulating hormone receptors, resulting in chronic follicular stimulation and hyperthyroidism (diffuse toxic goiter)
♦
Graves disease is most commonly seen in young females (20–40 years old; F:M = 4:1) and may occur in children. In the United States, Graves disease affects about one of every 2,000 people
♦
Patients present with diffuse goiter and hyperthyroidism. Patients often have a family history of autoimmune diseases. Graves disease is diagnosed clinically by physical examination and laboratory testing including elevated serum thyroxine with low TSH and high radioactive iodine uptake
♦
Untreated, Graves disease can cause severe thyrotoxicosis, osteoporosis, and catabolism of muscle and bone with myopathy, among other complication s
Macroscopic (Fig. 20.13)
♦
The thyroid shows mild to moderate diffuse enlargement and has soft consistency and pink tan color
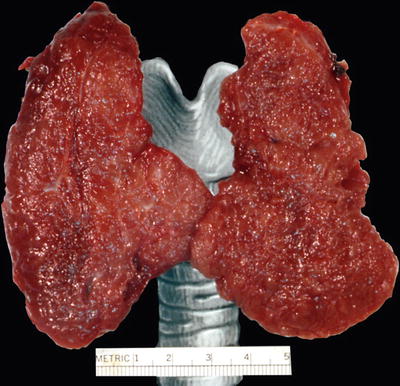
Fig. 20.13.
Graves disease: enlarged thyroid (94 g) with a glistening, finely nodular cut surface.
♦
The thyroid shows markedly hyperplastic follicles lined by active tall columnar cells showing clear, sometimes vacuolated, cytoplasm. Colloid appears pale and depleted, with scalloped edges at the epithelial border. Epithelial hyperplasia resulting in papillae with fibrovascular cores, resembling papillary carcinoma, is characteristically seen
♦
Interstitial lymphoid aggregates with germinal centers may be present
♦
The classic appearance is usually altered by preoperative medication. Iodine therapy can greatly diminish hyperplasia, resulting in an almost normal-appearing gland with involution of the epithelium with cuboidal rather than columnar cells, increased colloid, and decreased vascularity, while propylthiouracil and methimazole therapy are associated with hyperplastic changes
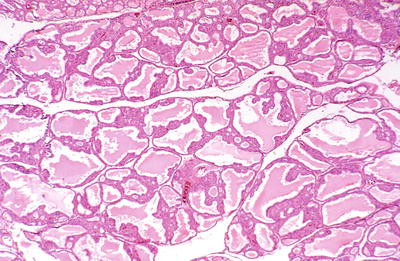
Fig. 20.14.
Graves disease : irregularly shaped follicles are partially filled with pale colloid.
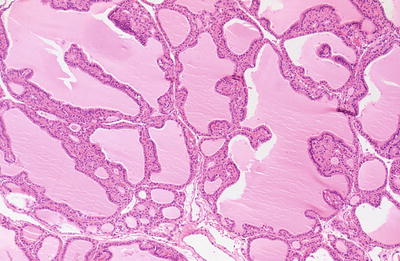
Fig. 20.15.
Graves disease: follicles show multiple papillary infoldings and some scalloping of the colloid.
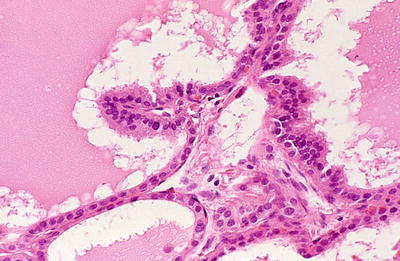
Fig. 20.16.
Graves disease : cubical and low columnar eosinophilic epithelium lines follicles, one of which has a papillary infolding.
Differential Diagnosis
♦
Papillary thyroid carcinoma
Papillary thyroid carcinoma generally presents as a mass, rather than a diffuse process. Histologically, classic cytologic features of papillary thyroid carcinoma (ground glass nuclei, nuclear grooves, and nuclear pseudo inclusions) are identified
In difficult cases of Graves disease with papillary hyperplasia, p27 protein and HBME-1 may be used to separate these two lesions. p27 shows higher expression in Graves disease compared with papillary thyroid carcinoma
♦
Lymphocytic thyroiditis
Lymphocytic thyroiditis usually affects young patients who are euthyroid. Thyroid follicles appear normal or show only mild atrophy when compared with the hyperplastic follicles classic of Graves disease
♦
Hashimoto thyroiditis
Hashimoto thyroiditis shows atrophy and Hürthle cell change, not hyperplasia
♦
Toxic nodular goiters
Toxic nodular goiters can also be associated with hyperthyroidism, but appear more multinodular than the diffuse appearance of the thyroid in Graves disease. Additionally, histologic involvement of the thyroid is focal in toxic nodular goiters, while it is diffuse in Graves disease
Nontoxic Simple and Multinodular Goiters
Clinical
♦
Goiter means enlarged thyroid gland. Simple nontoxic goiters can be sporadic or endemic. Sporadic nontoxic goiter is the most common nontoxic goiter. Sporadic goiters are identified in approximately 5% of the population, are more common in women, and are uncommon in children. The cause of sporadic goiters is generally unknown, but they can be associated with some medications and may have a hereditary component. When the thyroid cannot produce sufficient thyroid hormone, the gland enlarges to overcome this deficiency. Endemic goiters are seen in areas of iodine deficiency and have become relatively uncommon due to iodized table salt
♦
Most patients are euthyroid, presenting with symptoms of simple or multinodular thyroid enlargement, which may be generalized or have dominant nodule. Goiters may cause obstructive symptoms due to tracheal or esophageal compression and voice change due to laryngeal nerve compression. Goiters are usually slow growing and painless. A sudden increase in growth, a new nodule, or a dominant nodule in a multinodular goiter are concerning for malignancy. Goiters are treated with thyroxine, radioactive iodine, and surgery and can recur
Macroscopic (Fig. 20.17)
♦
The thyroid gland is enlarged in both simple and multinodular goiters. Simple goiters are diffusely enlarged, while multinodular goiters show multiple nodules grossly. The normal thyroid gland weighs 15–25 g, while goiterous thyroid glands can weigh over a 100 g
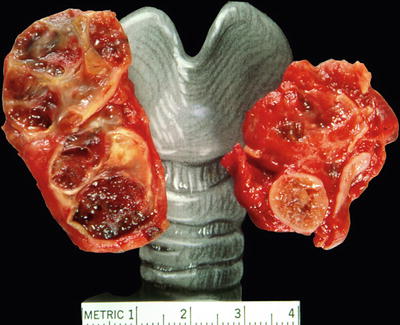
Fig. 20.17.
Multiple degenerating and hemorrhagic nodules.
Microscopic (Fig. 20.18)
♦
The histologic features of goiters can be varied. Goiters are generally composed of a mixture of small follicles with cuboid lining cells and large follicles with flattened lining cells. Large cystic structures showing hyperplastic areas of follicles or papillary structures (foci of secondary proliferation) are often seen
♦
Endemic and sporadic multinodular goiters show similar histologic features
♦
Multinodular goiters show many nodules of different sizes, some encapsulated, compress the adjacent thyroid parenchyma . An individual nodule, particularly a dominant nodule, in a multinodular goiter can be difficult to be distinguished from a follicular adenoma
♦
Degenerative changes with hemorrhage, fibrosis, and calcification are common
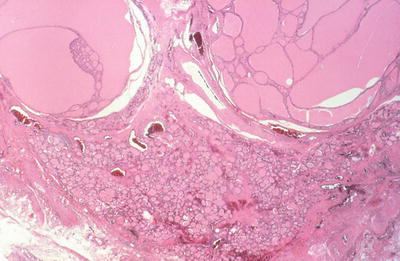
Fig. 20.18.
Macrofollicular nodule and colloid cyst.
Differential Diagnosis
♦
Follicular neoplasms
Follicular adenomas are difficult to be separated from a nodule of a multinodular goiter, particularly a dominant nodule. Multinodular goiters have multiple nodules, while follicular adenomas are more often single. However, clonality has been shown in adenomatous nodules. Follicular carcinoma arising in the setting of a multinodular goiter must show a clearly invasive growth pattern
Dyshormonogenetic Goiter
Clinical
♦
Dyshormonogenetic goiters are usually due to an autosomal recessive genetic defect in the synthesis of thyroid hormone. Although most dyshormonogenetic goiters develop in childhood, they can be identified over a wide age range. Patients present with hypothyroidism, goiter, or growth deficiencies. Patients with congenital goiter and congenital sensorineural deafness have a defect in the Pendred syndrome gene, 7q22–31.1, which encodes pendrin, an iodide/chloride transporter
Macroscopic
♦
Dyshormonogenetic goiters are large and multinodular
Microscopic
♦
The nodules are diffusely hypercellular and show an irregular growth pattern. The follicular cells show nuclear atypia and mitotic activity and can be confused with malignancy. Thyroid carcinomas, particularly follicular, can occur in these goiters
Toxic Multinodular Goiter
Clinical
♦
Toxic multinodular goiters are multinodular goiters associated with thyrotoxicosis, although these patients lack the ophthalmic changes seen in Graves disease. Multinodular goiters may be present for years and may insidiously become toxic goiters
Macroscopic
♦
Grossly toxic multinodular goiters appear similar to nontoxic multinodular goiters. Both are enlarged thyroids composed of multiple nodules with fibrosis
Microscopic
♦
Toxic multinodular goiters are composed of a mixture of hypercellular nodules with tall columnar cells, papillary hyperplasia, and inactive appearing nodules with flat epithelium
♦
The pattern of hypercellular nodules of toxic multinodular goiter differs from that of dyshormonogenetic goiters, which show more diffuse hyperplasia throughout the gland
Papillary Thyroid Carcinoma
Clinical
♦
Papillary thyroid carcinoma (PTC) is the most common thyroid carcinoma, accounting for approximately 80% of thyroid carcinomas. They occur in adults and children, with females being affected more commonly than males. Rare familial PTC can occur
♦
A variety of thyroid disorders including Graves, Hashimoto thyroiditis, and hyperplastic nodules have been identified with PTC. PTCs are associated with a history of radiation exposure, and radiation-associated tumors have been associated with aggressive behavior and solid growth pattern
♦
Papillary thyroid carcinomas are often irregular or well-demarcated masses on ultrasound and cold nodules on radioactive iodine scan
♦
The prognosis of PTC is generally excellent, with overall survival greater than 90%. Poor prognostic factors include increased age (>40 years), male sex, large tumor size, multicentricity, vascular invasion, extrathyroid extension, distant metastases, and dedifferentiation to poorly differentiated and anaplastic thyroid carcinoma. Variants of PTC with more aggressive behavior include tall cell, columnar cell, and solid variant
♦
Papillary thyroid carcinomas are often treated by complete thyroidectomy and ipsilateral lymph node dissection, and radioactive iodine can be used to ablate any remaining tumor
Macroscopic
♦
Papillary thyroid carcinomas are variably sized, from microscopic to >10 cm, usually 2–3 cm, solid, white, infiltrating masses, often with a granular cut surface and calcifications. These tumors may be single or multiple and may undergo cystic change
♦
Papillary thyroid carcinomas are composed of complex papillae with single layer or stratified epithelial cells supported by branching fibrovascular cores lined by follicular cells with characteristic cytologic features. Classic cases usually show a predominance of papillary structures, although this feature can be extremely variable. Foci of more follicular areas and solid areas as well as squamous metaplasia can be seen
♦
Diagnostic nuclear features include nuclear enlargement, nuclear irregularity, ground glass nuclei (large optically clear “Orphan Annie” nuclei that tend to overlap), nuclear grooves (due to folded nuclear membrane), and nuclear pseudoinclusions (due to intranuclear cytoplasmic invagination)
♦
Psammoma bodies (laminated basophilic bodies) occur in 50% of cases and are more common in tumors with prominent papillary growth
♦
The colloid in PTC is often darker than that of the surrounding normal thyroid
♦
The stroma is often abundant, fibrous, and sclerotic. A lymphocytic infiltrate can be seen at the periphery of the tumor lobules. Cystic change is common. Mitotic figures and necrosis are uncommon. A significant number of cases (25–75% depending on sampling) show multiple tumor foci
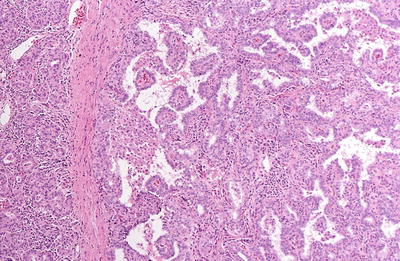
Fig. 20.19.
Papillary carcinoma : the tumor has papillary and follicular components separated by fibrous bands.
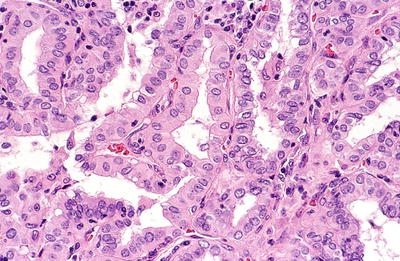
Fig. 20.20.
Papillary carcinoma : papillary processes with capillary cores mantled by medium-sized cells with eosinophilic cytoplasm and slightly irregular open nuclei with occasional grooves.
Immunohistochemistry
♦
Papillary thyroid carcinomas are positive for thyroglobulin, thyroid transcription factor (TTF1), and keratin (see Table 20.1). Papillary thyroid carcinomas are negative for chromogranin, synaptophysin, and calcitonin. Immunohistochemical markers that may be helpful confirming a diagnosis of PTC include HBME-1, galectin-3, cytokeratin 19, and CITED-1
Table 20.1.
Immunohistochemical Profile of Thyroid Tumors
Tumor | Thyroglobulin | TTF | Keratin | Calcitonin | Chromogranin | Synaptophysin |
|---|---|---|---|---|---|---|
Papillary thyroid carcinoma | + | + | + | − | − | − |
Follicular and Hürthle cell thyroid tumors | + | + | + | − | − | − |
Poorly differentiated thyroid carcinoma | + | + | + | − | − | − |
Anaplastic thyroid carcinoma | Focal | Focal | Focal | − | − | − |
Medullary thyroid carcinoma | − | + | + (CAM 5.2) | + | + | + |
Molecular Genetics
♦
BRAF mutations and RET/PTC rearrangements are alternative events in the pathogenesis of PTC
♦
BRAF mutations , especially BRAF(V600E), is detected in 36–69% of PTC. BRAF mutations are frequent in PTC with papillary or mixed papillary–follicular growth pattern, Warthin-like PTC, micropapillary PTC, and oncocytic/oxyphilic (Hürthle cell) variant of PTC. BRAF mutations are rarely detected in pediatric or radiation-associated PTC. BRAF mutations are also present in poorly differentiated and anaplastic carcinomas arising from PTC
♦
RET/PTC rearrangement is identified in approximately 20–30% of PTC, although the prevalence varies among geographical sites and tumor subtypes. This rearrangement is particularly common in radiation-associated PTC. RET/PTC1 and RET/PTC3 account for greater than 90% of the rearrangements. RET/PTC1 is more common in papillary microcarcinomas, tumors with classic papillary growth, and a more benign clinical course, while RET/PTC3 is more common in the solid variant of PTC and aggressive behavior
Variants
♦
Papillary microcarcinoma
Papillary microcarcinoma is defined as a PTC measuring 1 cm or less. These tumors are usually indolent, incidental findings in thyroids removed for benign clinical nodules or thyroditis. Autopsy and surgical studies show that approximately 8% of thyroids when serially sectioned will have a papillary microcarcinoma
The treatment for papillary microcarcinoma has been controversial. Papillary microcarcinomas presenting as a clinical mass or a lymph node metastasis are treated as a clinical cancer. In up to 15–20% of papillary microcarcinomas, another focus is the contralateral lobe, and that long-term recurrent disease may reach 20% in the absence of complete resection. Despite their small size, 5% of these microcarcinomas may be associated with invasion of the capsule or distant metastases. When papillary microcarcinomas present as a clinically occult incidental finding, the behavior appears more indolent than those presenting clinically, and some suggest a conservative approach to management
♦
Follicular variant of PTC
Follicular variant of PTC (FVPTC ) is the most common variant of PTC and is also the most difficult type to diagnose. Encapsulated tumors can be particularly problematic if the tumor shows only borderline features for PTC. These tumors can show a microfollicular, macrofollicular, or diffuse growth pattern. Differentiating FVPTC from the follicular neoplasms can be extremely difficult. Cytologic features classic of PTC may only be focally noted in FVPTC. Important diagnostic features of FVPTC are nuclear pseudoinclusions (cytoplasmic invaginations into the nucleus), abundant nuclear grooves, and ground glass nuclei. Immunohistochemical studies such as HBME-1, CK19, galectin, and CITED are helpful in separating some cases of FVPTC from follicular adenoma. Encapsulated FVPTC may have more indolent behavior than those that are unencapsulated or those that have invasive growth
♦
Tall cell variant of PTC
The tall cell variant of PTC is more common in the elderly and in males. These tumors are usually large at diagnosis. Histologically, prominent papillary structures are lined by cells that are least twice as tall as they are wide. The tall cells have basally located nuclei and abundant eosinophilic cytoplasm. The tall cell variant of PTC is associated with extrathyroidal disease, vascular invasion, recurrence, and metastases. These are aggressive tumors with shorter disease-free survival than conventional PTC and have a fatality rate of up to 25%
♦
Columnar variant of PTC
The columnar variant of PTC is an uncommon, aggressive variant of PTC that occurs over a wide age range, can metastasize widely, and usually does not respond to radioactive iodine or chemotherapy. The columnar variant has a prominent papillary architecture and elongated cells with nuclear stratification and scant cytoplasm
♦
Solid variant of PTC
The solid variant of PTC is uncommon, comprising only 2–3% of PTC, is more common in children, and may be associated with radiation exposure. This variant has a less favorable prognosis than classic PTC
♦
Diffuse sclerosing variant of PTC
The diffuse sclerosing variant of PTC is more common in women and younger patients. This variant is characterized by diffuse thyroid involvement, intrathyroid lymphatic spread, squamous metaplasia, prominent psammoma bodies, fibrosis, and a lymphocytic infiltrate. This variant shows frequent cervical lymph node as well as lung metastases. Some studies have found decreased survival, while others have shown similar to survival to classic PTC
♦
Oxyphilic (oncocytic/Hürthle cell variant of PTC)
The oxyphilic (oncocytic/Hürthle cell) variant of PTC is uncommon. These tumors often show a papillary architecture and have oxyphilic cytoplasm and nuclear features classic of PTC. This variant may behave similarly to or more aggressively than classic PTC, depending on the study
♦
Warthin-like variant of PTC
The Warthin-like variant of PTC resembles a Warthin tumor of the salivary gland with oxyphilic cells and lymphocytic stroma. However, the cytologic features are classic of PTC, and these tumors behave similarly to classic PTC
♦
Cribriform-morular variant of PTC
The cribriform-morular variant of PTC can occur sporadically, but often occurs in the setting of familial adenomatous polyposis with germ line APC gene mutations. These tumors are often multicentric and are more common in women. These tumors show cribriform, solid/trabecular, and morular growth and cytologic features classic of PTC. These tumors behave similarly to conventional PTC
♦
Clear cell variant of PTC
The clear cell variant of PTC is uncommon and behaves similar to conventional PTC. This variant must be differentiated from other clear cell tumors such as metastatic renal cell carcinoma
♦
Papillary thyroid carcinoma with nodular fasciitis-like stroma
Rarely, PTC shows prominent nodular fasciitis-like stroma that can be mistaken for a mesenchymal tumor or an anaplastic thyroid carcinoma. Small tubules and nests of epithelioid cells with cytologic features characteristic of PTC are noted in a nodular fasciitis-like stroma
♦
Papillary thyroid carcinoma with lipomatous stroma
Papillary thyroid carcinoma can show a lipomatous stroma. Lipomatous stroma is not specific for PTC as it can also be seen in adenomatous nodules, follicular neoplasms, goiters, and lymphocytic thyroiditis
♦
Hobnail variant of PTC
The recently described hobnail variant of PTC is composed of prominent papillae lacking vascular cores and follicles lacking colloid. The lining cells have prominent hobnail (micropapillary) features with high nuclear to cytoplasmic ratios and apical nuclei which give the tumor a hobnail appearance. The hobnail variant of PTC appears to be much more aggressive than other types of PTC with up to half of patients with tumors with >30% hobnail component dying of disease. Even tumors with <30% hobnail component may be associated with aggressive behavior
Differential Diagnosis
♦
Graves disease
Graves disease can show papillary architectural features, but it lacks typical nuclear features such as nuclear clearing, large nuclei with irregular nuclear membranes, nuclear clearing (“Orphan Annie” nuclei), nuclear grooves, and intranuclear holes that are helpful in separating PTC from Graves
♦
Hyalinizing trabecular tumor (Figs. 20.21 and 20.22)
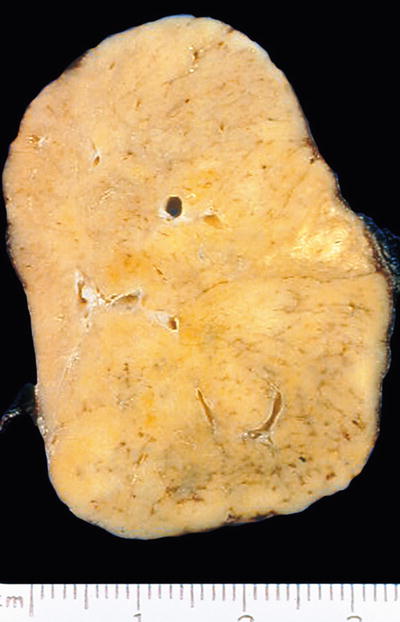
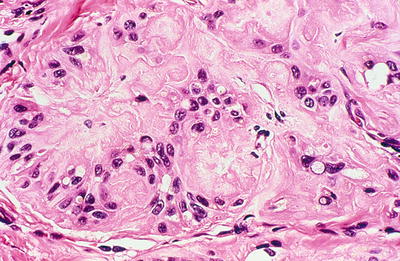

Fig. 20.21.
Hyalinizing trabecular adenoma : well-circumscribed, solid, tan tumor.

Fig. 20.22.
Hyalinizing trabecular adenoma : abundant hyaline material. Nuclei are polymorphic, many are oval. Intranuclear and cytoplasmic inclusions are present.
The nuclei in hyalinizing trabecular tumors are similar to those of PTC, and occasional psammoma bodies can be seen in hyalinizing trabecular tumors. However, hyalinizing trabecular tumors often resemble neuroendocrine neoplasm (paraganglioma or medullary thyroid carcinoma). Hyalinizing trabecular tumors are characterized by trabecular growth pattern with prominent intratrabecular hyaline material. Hyalinizing trabecular tumors show a characteristic cytoplasmic staining pattern for MIB1 (Ki67) , which can also be helpful in their diagnosis
♦
Follicular carcinoma
Follicular thyroid carcinomas may resemble PTC, particularly FVPTC. Follicular thyroid carcinomas do not show papillary architecture or classic cytologic features (nuclear grooves, intranuclear cytoplasmic inclusions) of PTC
♦
Follicular adenoma
Follicular adenomas may resemble PTC, particularly FVPTC. Follicular adenomas do not show papillary architecture, invasive growth, or classic cytologic features (nuclear grooves, intranuclear cytoplasmic inclusions) of PTC. Cytologic features classic of PTC may only be focally noted in FVPTC often at the peripheral aspect of the tumor. Important features to diagnose FVPTC are nuclear pseudoinclusions (cytoplasmic invaginations into the nucleus), abundant nuclear grooves, and ground glass nuclei. Immunoperoxidase studies, including HBME-1, galectin-3, and cytokeratin 19, can also be helpful in difficult cases as PTC are usually positive
♦
Hürthle cell tumors
The Warthin-like variant and oxyphilic variant of PTC can be mistaken for benign Hürthle cell tumors. Cytologic features classic of PTC are helpful in separating the Warthin-like variant of PTC from Hürthle cell tumors. The prominent lymphoplasmacytic infiltrate seen in Warthin-like variant of PTC is a low power clue to the diagnosis
Hyalinizing Trabecular Tumor
Clinical
♦
Hyalinizing trabecular tumors are unique and can mimic PTC and medullary thyroid carcinoma . The biologic potential has been controversial, but the overwhelming majority of these tumors behave in an indolent fashion. They should probably only be treated as a malignancy when there is obvious capsular or vascular invasion or metastasis
Macroscopic
♦
Hyalinizing trabecular tumors are circumscribed, yellow-tan, encapsulated nodules that measuring 0.3–4 cm in diameter
♦
Hyalinizing trabecular tumors are circumscribed or encapsulated solid tumors that show a trabecular growth pattern with prominent extracellular eosinophilic hyaline fibrosis that is reminiscent of amyloid but negative for Congo red
♦
The trabeculae and nests of cells are composed of polygonal and elongated cells with oval nuclei with perinuclolar vacuoles, nuclear inclusions, nuclear grooves, and fine granular cytoplasm. The prominent intranuclear cytoplasmic inclusions can cause confusion with PTC
♦
Cytoplasmic yellow bodies are frequently noted in hyalinizing trabecular tumors, but these are not specific as they can be seen in other tumors
Immunohistochemistry
♦
Hyalinizing trabecular tumors are positive for thyroglobulin and TTF1 and negative for calcitonin, chromogranin, and CEA. These tumors also show characteristic cytoplasmic and cell membrane staining for MIB1 (Ki67)
♦
Galectin-3 shows variable staining. HBME-1, a marker that is usually positive in PTC, is negative in hyalinizing trabecular tumors
Molecular Genetics
♦
RET proto-oncogene rearrangements are a common finding in PTC and have been detected by RT-PCR and immunohistochemistry in some cases. However, some of the cases also harbored PTC; thus this finding is difficult to interpret
♦
BRAF mutations are generally not detected in hyalinizing trabecular tumors
Differential Diagnosis
♦
Medullary thyroid carcinoma
The trabecular growth pattern, elongated cells, and hyalinization, which are reminiscent of amyloid, are features of hyalinizing trabecular tumor that can be confused with medullary thyroid carcinoma. However, the hyaline material, unlike amyloid, is negative for Congo red. Cytologically, the nuclear features of hyalinizing trabecular tumors are more similar to PTC rather than the neuroendocrine nuclear features seen in medullary thyroid carcinoma. The immunophenotype is also helpful in differentiating these tumors as hyalinizing trabecular tumors are positive for thyroglobulin and negative for chromogranin, synaptophysin, and calcitonin
♦
Papillary thyroid carcinoma
Hyalinizing trabecular tumors show prominent nuclear inclusions, a feature often seen in PTC. However, the nuclear inclusions are much more prominent and numerous in hyalinizing trabecular tumors. Additionally, PTC generally lacks the trabecular growth pattern and prominent stromal hyalinization characteristic of hyalinizing trabecular tumors. HBME-1 is generally positive in PTC and negative in hyalinizing trabecular tumors. Hyalinizing trabecular tumors also show characteristic cytoplasmic membrane staining with MIB1 (Ki67)
Follicular Adenoma
Clinical
♦
Follicular adenomas are benign, encapsulated tumors showing follicular differentiation
♦
Follicular adenomas occur in 5–10% of the population, predominantly in adults, and are more common in women. Patients present with a palpable nodule showing little to no I131 uptake or as asymmetrical thyroid enlargement
Macroscopic
♦
Follicular adenoma is a single encapsulated nodule, 1–3 cm in size. The cut surface varies from gray-tan to pink and fleshy, depending on cellularity and colloid. Larger adenomas may show hemorrhage, cyst formation, fibrosis, and calcification
Microscopic
♦
Follicular adenomas have a complete, usually thin, capsule with compression of surrounding thyroid tissue. No invasion of the capsule or vessels is present
♦
A variety of growth patterns may be seen within the nodule including microfollicular, macrofollicular, trabecular, solid, and focal pseudopapillary architecture, which can be seen occasionally (Figs. 20.23 and 20.24). The growth pattern of cells within nodule classically stands in sharp contrast to appearance of normal thyroid parenchyma outside capsule
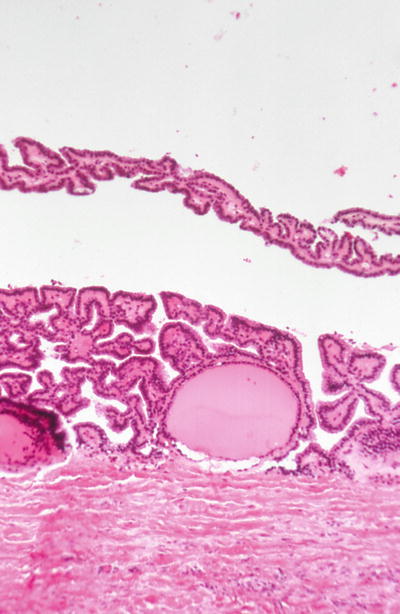
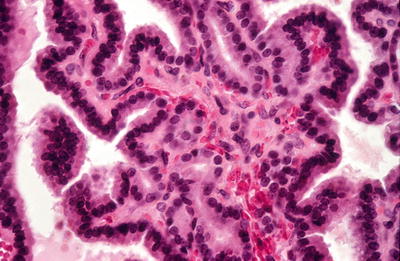

Fig. 20.23.
Cystic adenoma surrounded by a thick fibrous capsule.

Fig. 20.24.
Adenoma: papillations are lined by a uniform epithelium with basal hyperchromatic nuclei.
♦
Many histologic variants of follicular adenoma have been described as follicular adenoma with bizarre nuclei, adenolipomas, adenochondromas, mucinous follicular adenomas, toxic adenomas, follicular adenomas with clear cell change, follicular adenoma with papillary hyperplasia, and signet ring cell adenomas
♦
Atypical follicular adenomas are cellular tumors, which may show nuclear atypia or irregular growth, but lack definitive vascular or capsular invasion. Atypical adenomas usually behave in an indolent fashion
Immunohistochemistry
♦
Follicular adenomas are immunoreactive for thyroglobulin, keratin, and TTF1
♦
Follicular adenomas generally do not show prominent staining for HBME-1, CK19, galectin 3, and CITED as is often seen in PTC
Molecular Genetics
♦
Specific genetic markers diagnostic of follicular adenoma have not been identified. Follicular adenomas frequently show RAS mutations, and a small percentage of tumors may show PAX8 to PPAR gamma (peroxisome proliferator-activated receptor gamma) rearrangements
Differential Diagnosis
♦
Follicular thyroid carcinoma
By definition follicular thyroid carcinomas show capsular or vascular invasion, features not seen in follicular adenomas. The capsule in follicular adenomas is usually not as thick as in follicular carcinoma
♦
Follicular variant of PTC
Differentiating follicular adenoma from FVPTC can be extremely difficult as the tumors show architectural similarities. In most cases, FVPTC shows cytologic features classic of PTC, but classic cytologic features of PTC may only be focally noted often near the capsule in FVPTC. Thus, careful histologic evaluation is critical to differentiate these entities. Immunohistochemical markers somewhat helpful in this distinction include HBME-1, CK19, and galectin 3
♦
Adenomatous nodule
Adenomatous nodules are usually multiple, but may present as a dominant nodule. Adenomatous nodules are often not completely encapsulated. Prominent secondary hyperplastic papillary changes can be seen
♦
Hyalinizing trabecular tumor (Figs. 20.21 and 20.22)
Hyalinizing trabecular tumors with trabecular growth and prominent hyaline material are very helpful in identification of these tumors. The nuclei are similar to PTC, and numerous intranuclear holes can be seen. Hyalinizing trabecular tumor shows a characteristic cytoplasmic membrane staining pattern for MIB1 (Ki67)
Follicular Thyroid Carcinoma
Clinical
♦
Follicular c arcinoma is a malignant epithelial tumor showing follicular differentiation and lacking the diagnostic nuclear features of PTC
♦
Follicular carcinomas account for 10–15% of thyroid carcinomas, present as a single palpable neck mass, affect females more commonly than males, and occur at an average age of 50 years. A higher incidence of follicular carcinomas is identified in iodine-deficient regions. These tumors show little or no I131 uptake
♦
Unlike PTC, follicular carcinomas show infrequent regional lymph node metastases
♦
Patients with follicular thyroid carcinomas usually undergo total thyroidectomy. Minimally, invasive follicular carcinomas have an excellent prognosis with a mortality of 5%, while widely invasive carcinomas have a mortality of 50%
Macroscopic
♦
Follicular carcinomas are solid, round to oval tumors, tan to brown in color. These tumors may grossly appear encapsulated or may form a poorly circumscribed mass. The capsule is often thicker and more irregular than in follicular adenoma
♦
Widely invasive tumors may show gross capsular or vascular invasion. The cut surface usually appears similar to follicular adenoma (gray-tan to pink and fleshy)
♦
Histologically, follicular carcinoma can be difficult to distinguish from follicular adenoma. Follicular carcinomas are cellular, show a follicular or solid growth pattern, and lack cytologic features of PTC
♦
Vascular invasion or capsular invasion is required for the diagnosis of follicular carcinoma. Capsular invasion often shows a mushroom-type of growth through the capsule. This pattern is different from the linear pattern of tumor associated with hemorrhage, inflammation, and young collagen that is seen in fine-needle aspiration sites
♦
Follicular carcinomas have been classified as minimally and widely invasive based on the amount and type of invasion. Minimally invasive tumors show focal capsular and/or vascular invasion, while widely invasive carcinomas show many areas of invasion. Tumors with capsular invasion only (no vascular invasion) may behave less aggressively than those with vascular invasion. Tumors with prominent vascular invasion may also be referred to as angioinvasive follicular carcinomas
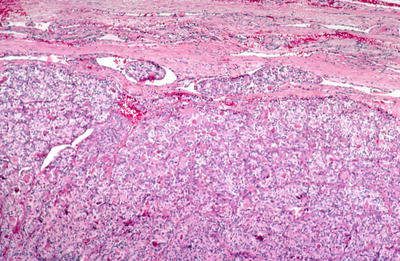
Fig. 20.25.
Follicular carcinoma : the tumor shows invasion of a capsular blood vessel.
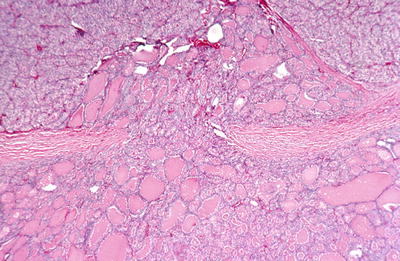
Fig. 20.26.
Follicular carcinoma : the tumor has mushroomed through the wall of its fibrous capsule.
Immunohistochemistry
♦
Follicular carcinomas are positive for thyroglobulin, TTF1, and low molecular weight cytokeratin (CAM 5.2) (Table 20.1)
Molecular Genetics
♦
PAX8-PPAR gamma translocation t(2;3)(q13;p25) resulting in a fusion oncoprotein has been identified in a proportion of follicular carcinomas and occasionally in follicular adenomas. Follicular carcinomas with PAX8-PPAR gamma occur in younger patients with smaller, but overtly invasive, tumors, which are usually positive for galectin-3 and negative for HBME-1
♦
RAS mutations appear separate from PAX8-PPAR gamma translocations. Follicular carcinomas with RAS mutations are often positive for HBME-1 and negative for galectin-3
Differential Diagnosis
♦
Follicular adenoma
Follicular carcinomas show similar encapsulation and growth patterns as follicular adenomas, but follicular carcinomas by definition show capsular and/or vascular invasion. The capsules of adenomas tend to be thinner than carcinomas
♦
Papillary carcinoma, follicular variant
Follicular variant of PTC may show invasive growth; however, cytologic features of PTC are present. Often, focal papillary differentiation is noted
Hürthle Cell Adenoma
Clinical
♦
Hürthle cell adenomas are benign thyroid neoplasms composed exclusively or predominantly of Hürthle cells (oxyphil cells, oncocytes). Women are affected more commonly than men. The mean age at diagnosis is 55 years. These tumors are solitary nodules on ultrasound and show little or no I131 uptake
Macroscopic (Fig. 20.27)
♦
Hürthle cell adenomas are solid tumors, round to oval in shape, encapsulated, and have a brown cut surface typical of oncocytic tumors. Areas of hemorrhage, cyst formation, or calcification may be seen in larger tumors. Infarction can be seen in tumors that have undergone fine-needle aspiration biopsy
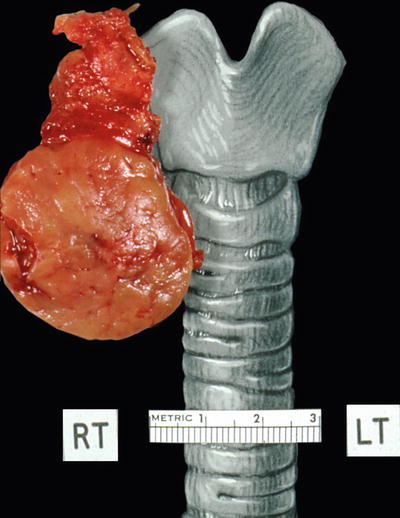
Fig. 20.27.
Oxyphilic adenom a: circumscribed slightly lobulated tan-colored tumor.
♦
Hürthle cell adenomas are encapsulated and lack capsular or vascular invasion
♦
The tumors can show a variety of growth patterns including follicles, solid areas, trabecular growth, and papillary growth. They are composed of at least 75% Hürthle cells, which are polygonal cells with abundant granular eosinophilic cytoplasm and large nuclei with prominent nucleoli. Areas of nuclear pleomorphism (“endocrine atypia”) may be identified
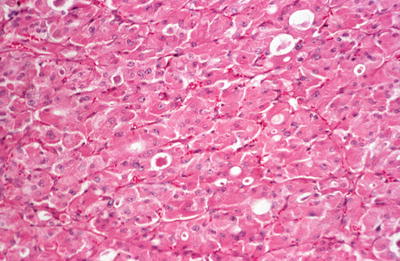
Fig. 20.28.
Oxyphilic adenoma : solid tumor composed of cells with eosinophilic cytoplasm that shows some follicle formation.
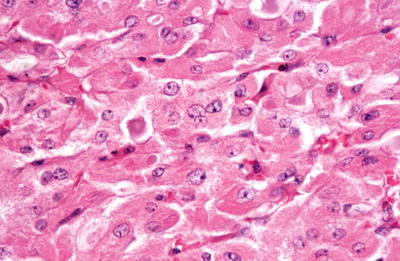
Fig. 20.29.
Oxyphilic adenoma: large, poorly demarcated cells with granular eosinophilic cytoplasm and vesicular nuclei with prominent nucleoli.
Immunohistochemistry
♦
Hürthle cell adenomas are positive for thyroglobulin and TTF1 and are negative for chromogranin, synaptophysin, and calcitonin
Differential Diagnosis
♦
Hürthle cell carcinoma
Hürthle cell adenomas lack capsular and vascular invasion of carcinomas
♦
Hyperplastic Hürthle cell adenomatous nodule in Hashimoto thyroiditis
♦
Adenomatous Hürthle cell nodules are multiple, lack a well-defined capsule, and are associated with a prominent lymphoplasmacytic infiltrate
♦
Hürthle cell/oxyphil/oncocytic variant of PTC
♦
Hürthle cell adenomas lack cytologic features classic of PTC
Hürthle Cell Carcinoma
Clinical
♦
Hürthle cell carcinomas are malignant neoplasms composed predominantly or exclusively of Hürthle (oncocytic/oxyphilic) cells
♦
Hürthle cell carcinoma accounts for 2–3% of thyroid carcinomas. Women are more commonly affected than men. The mean age at diagnosis is 55 years. Hürthle cell carcinoma is a well-demarcated nodule on ultrasound and shows little or no I131 uptake
♦
Hürthle cell carcinomas are slightly more aggressive than follicular carcinomas and have an overall 5-year survival of 50–60%. Hürthle cell carcinomas show extrathyroidal invasion more frequently than follicular carcinomas and show lymph node metastases more often than follicular carcinomas. However, the most common sites of metastases are lung and bone, followed by regional lymph nodes. Poor prognostic factors include large tumor size and vascular invasion
Macroscopic
♦
Hürthle cell carcinomas are solid, encapsulated, round to oval tumors, with a brown cut surface. They are generally larger than adenomas and have a thicker more irregular capsule. Widely invasive tumors may show gross invasion of the capsule or vessels
Microscopic
♦
Hürthle cell carcinomas are cellular tumors, composed of at least 75% Hürthle cells, and show a follicular, solid, or trabecular growth pattern. Hürthle cells are large polygonal cells with round nuclei and nucleoli and abundant granular eosinophilic cytoplasm and lack cytologic features of papillary thyroid carcinoma
♦
Vascular or capsular invasion is required for the diagnosis of Hürthle cell carcinoma. Capsular invasion often shows a mushroom type of growth through the capsule. This pattern is different from the linear pattern of tumor associated with hemorrhage, inflammation, and young collagen that is seen in fine-needle aspiration sites. Hürthle cell carcinomas have been classified as minimally or widely invasive based on the amount and type of invasion. Minimally invasive tumors show focal capsular and/or vascular invasion, while widely invasive follicular carcinomas show many areas of invasion
Immunohistochemistry
♦
Hürthle cell carcinomas are immunoreactive for thyroglobulin, although staining is not as strong as other thyroid follicular neoplasms (Table 20.1). Hürthle cell carcinomas are also positive for TTF1 and are negative for chromogranin, synaptophysin, and calcitonin
Molecular Genetics
♦
Numeric chromosomal abnormalities include changes in p53 and cyclin D1 and chromosomal gains and have been identified in Hürthle cell carcinomas. These tumors tend to show more chromosomal losses than adenomas. PAX8-PPAR gamma rearrangements and RAS mutations are not frequent in Hürthle cell carcinomas. GRIM-19 point mutations were the first mutation relatively specific to Hürthle cell tumors
Differential Diagnosis
♦
Hürthle cell adenoma
Hürthle cell adenomas are usually smaller, have a thinner more regular capsule, and lack diagnostic capsular and vascular invasion of Hürthle cell carcinomas
♦
Hürthle cell/oxyphilic variant of PTC
Hürthle cell carcinomas lack the classic cytologic features of PTC
♦
Oxyphilic variant of medullary thyroid carcinoma
Hürthle cell carcinomas lack the neuroendocrine nuclear features of medullary thyroid carcinoma. In difficult cases, immunohistochemical studies can be helpful as Hürthle cell carcinomas lack staining for chromogranin, synaptophysin, and calcitonin
Poorly Differentiated Carcinoma
Clinical
♦
Poorly differentiated carcinomas are follicular cell neoplasms that are in between well-differentiated follicular and papillary carcinomas and undifferentiated or anaplastic carcinomas
♦
Patients usually present with a large solitary thyroid mass . They often have a history of recent thyroid enlargement or enlargement of a tumor that has been present for a number of years. Patients are usually >50 years of age, and women are affected more commonly than men. Poorly differentiated thyroid carcinomas appear to be more common in Italy and Latin America
♦
Treatment is total thyroidectomy with resection of any involved lymph nodes and radioactive iodine therapy. The prognosis is relatively poor with lymph node, with distant metastases being relatively common. The 5-year survival rate is approximately 50%
Macroscopic
♦
Poorly differentiated tumors are large, have a pale, gray-white cut surface with areas of necrosis and may have a pushing border, extrathyroid extension, and satellite nodules
Microscopic
♦
These tumors can show a variety of growth patterns including insular, trabecular, and solid. Some authors refer to tumors showing an insular or trabecular growth pattern growth as “insular carcinomas.” Tumors may also show a characteristic “peritheliomatous” pattern of growth with extensive necrosis with preservation of viable cells around vessels. The tumor cells may show nesting or sheetlike growth with necrosis and vascular invasion frequently identified. The tumor cells are small and uniform with vesicular or hyperchromatic nuclei, small nucleoli, and prominent mitotic activity. Areas resembling papillary or follicular carcinomas are identified in association with some tumors, raising the possibility that these tumors may have dedifferentiated. Recent studies have suggested diagnostic criteria of solid/trabecular/insular growth, absence of papillary thyroid carcinoma cytologic features, and the presence of one of the following criteria (convoluted nuclei or 3 or more mitoses per 10 high power fields or tumor necrosis). Others find high-grade features, mitotic index, and necrosis to be most helpful diagnostically regardless of growth pattern
Immunohistochemical
♦
These tumors are positive for thyroglobulin, low molecular weight kininogen, and TTF (see Table 20.1). If present, TP53 nuclear staining is usually focal and high Ki67 index is common
Molecular Genetics
♦
Mutations of H-, K-, and N-RAS are identified in 50% of poorly differentiated carcinomas. Mutations of TP53 mutations are present in 20–30%, and p53 overexpression is present in 40–50%. A small percentage of tumors may show BRAF mutation, RET/PTC, or NTRK1 rearrangements
Differential Diagnosis
♦
Medullary thyroid carcinoma
Medullary thyroid carcinomas show neuroendocrine differentiation with stippled chromatin and are positive for chromogranin, synaptophysin, and calcitonin
♦
Follicular carcinoma
Follicular carcinomas lack the nuclear atypia, prominent mitotic figures, and necrosis seen in poorly differentiated thyroid carcinoma
♦
Solid variant of PTC
Poorly differentiated carcinomas often show prominent mitotic activity and areas of necrosis and lack the cytologic features classic of PTC
♦
Metastatic carcinomas to the thyroid
Poorly differentiated thyroid carcinomas are positive for TTF1, a marker helpful in differentiating primary thyroid carcinomas from metastases, except those from the lung which also show positivity for TTF1
Anaplastic Thyroid Carcinoma
Clinical
♦
Anaplastic (undifferentiated) thyroid carcinoma is a highly malignant tumor composed in part or entirely of undifferentiated cells with immunohistochemical and ultrastructural features supporting epithelial differentiation
♦
These tumors usually present as a rapidly expanding mass with symptoms of regional invasion (dysphagia, dyspnea, or dysphonia) in elderly patients. Females are affected more commonly than males (1.5:1)
♦
At surgery, invasion of adjacent structures including the neck muscles, trachea, esophagus, laryngeal nerve, and larynx is common. Almost half of all patients have distant metastases at diagnosis. The most common metastatic sites include the lung, bone, and brain. These are highly aggressive tumors with a mortality rate of greater than 90% and a mean survival of 6 months
Macroscopic (Fig. 20.30)
♦
The tumors are grossly invasive, replace the thyroid, and extensively involve regional neck structures. The tumors have a white to tan cut surface and are firm to hard. Hemorrhage and necrosis are common
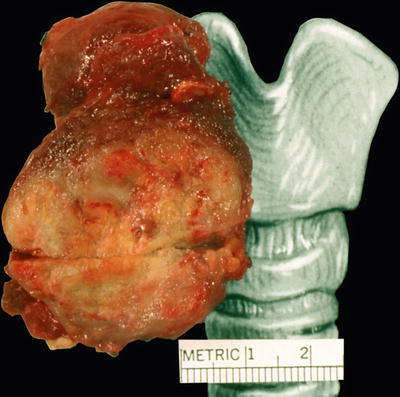
Fig. 20.30.
Anaplastic carcinoma : the tumor occupies most of the right lobe of thyroid.



