Thyroid
The term ‘goitre’ simply denotes enlargement of the thyroid gland. It may be diffuse or nodular, simple or toxic, benign or malignant, and physiological or pathological. Minor enlargement of the thyroid is common, especially in women. Both hypothyroidism and hyperthyroidism are also common. In contrast, thyroid cancer is relatively rare.
Non-Toxic Goitre
Aetiology
A variety of factors may predispose to thyroid enlargement:
- Simple goitre: iodine deficiency, especially in areas of endemic goitre. Sporadic goitre may reflect relative iodine deficiency for that patient. Iodine requirement is increased at puberty in girls and during pregnancy.
- Goitrogens: e.g. iodide in large doses, antithyroid drugs, lithium.
- Inborn errors of thyroid hormone biosynthesis (dyshormonogenesis): the production of thyroid hormones is mediated by iodide uptake and oxidation, organification of thyroglobulin to generate iodotyrosines followed by their coupling to yield thyroxine (T4) and triiodothyronine (T3). Several genetic disorders involving proteins in this biosynthetic pathway have been described. For example, Pendred syndrome, which is characterised by sensorineural deafness and goitre, is caused by defects in pendrin, which transports iodine into the follicular lumen.
Clinical Presentation
The patient (or a relative) usually notices a painless swelling of the thyroid. With time it may develop into a large nodular goitre and cause pressure on the trachea, oesophagus or veins, especially if there is significant retrosternal extension.
Differential Diagnosis
The differential diagnosis of thyroid enlargement is shown in Table 16.1.
Table 16.1 Causes of Thyroid Enlargement (Goitre and/or Nodules)
| Type | Examples | |
| Diffuse goitre | Physiological | Puberty, pregnancy |
| Autoimmune | Graves’ disease, Hashimoto’s disease | |
| Thyroiditis | Sub-acute (De Quervain’s), Riedel’s | |
| Endemic | Iodine deficiency | |
| Goitrogens | Antithyroid drugs, lithium, iodine excess | |
| Dyshormonogenesis | Pendred syndrome | |
| Nodular goitre | Multinodular | Toxic, non-toxic |
| Solitary nodule | Follicular adenoma, benign nodule or cyst, thyroid malignancy, lymphoma, metastasis | |
| Infiltration (rare) | Tuberculosis, sarcoidosis. |
Investigation
- Thyroid function tests: serum-free thyroxine (FT4) and thyrotropin (thyroid-stimulating hormone, TSH); if TSH is suppressed, but with normal FT4, check serum-free triiodothyronine (FT3) for possible T3-toxicosis.
- Thyroid autoantibodies (e.g. antithyroid peroxidase) for Hashimoto’s disease.
- CT scan of neck and thorax if pressure symptoms are present; respiratory flow-volume loops may also help to confirm/refute significant airway obstruction.
- Ultrasound can help distinguish solid or cystic masses and whether single or multiple nodules but is not required in all cases.
- Fine-needle aspiration biopsy (FNAB) with cytology may be required for solitary or dominant nodules or if there is clinical concern regarding possible malignancy.
NB Thyroid radioisotope scans are not generally performed unless the patient is thyrotoxic (see below).
Treatment
If the patient is euthyroid and there are no concerns regarding possible malignancy, treatment is not required unless the swelling is unsightly or causing pressure symptoms, when surgery (or occasionally radioiodine therapy) may be indicated. If TSH is raised (subclinical hypothyroidism), thyroxine can be given to suppress TSH hypersecretion (TSH is trophic for thyroid growth).
Thyrotoxicosis (Hyperthyroidism)
Thyrotoxicosis is the clinical disorder resulting from exposure to raised circulating levels of thyroid hormone (T4 and/or T3). It is most commonly due to thyroid gland dysfunction (hyperthyroidism), but can occur when exogenous T4 and/or T3 is taken in excess. The prevalence of hyperthyroidism in the UK has been estimated at 1–2%, with a female predominance female : male 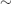 5–10 : 1.
5–10 : 1.
Aetiology
The causes of thyrotoxicosis are shown in Table 16.2. Graves’ disease is an autoimmune disorder in which autoantibodies against the TSH receptor (TRAb) provide continual (unregulated) stimulation of thyroid follicular cells leading to thyroid enlargement (diffuse smooth goitre) and thyrotoxicosis. Involvement of the retro-orbital tissues and skin may also result in Graves’ ophthalmopathy and dermopathy (pretibial myxoedema). Environmental ‘triggers’ (e.g. stress, pregnancy, drugs) combined with genetic susceptibility (e.g. HLA-DR3, cytotoxic T-lymphocyte antigen 4 (CTLA4) variants) are implicated in many cases.
Table 16.2 Causes of Thyrotoxicosis
| Type | Frequency | Condition(s) |
| Primary | Common | Graves’ disease |
| Toxic multinodular goitre | ||
| Less common | Solitary toxic adenoma | |
| Thyroiditis (e.g. post-partum; subacute) | ||
| Drug-induced (e.g. amiodarone; over-treatment with/inappropriate administration of L-T4 and/or L-T3 therapy) | ||
| Excess iodine intake (e.g. health food supplements such as kelp, or drugs, e.g. amiodarone) | ||
| ‘Hashitoxicosis’ (hyperthyroid phase of Hashimoto’s thyroiditis) | ||
| Rare | Metastatic follicular thyroid carcinoma | |
| Struma ovarii | ||
| Secondary | Rare | TSH-secreting pituitary adenoma |
| Resistance to thyroid hormone | ||
| Trophoblastic tumour secreting hCG (shared/common α sub-unit with TSH) | ||
| TSH, thyrotropin (=thyroid-stimulating hormone) hCG, human chorionic gonadotrophin. | ||
Clinical Presentation
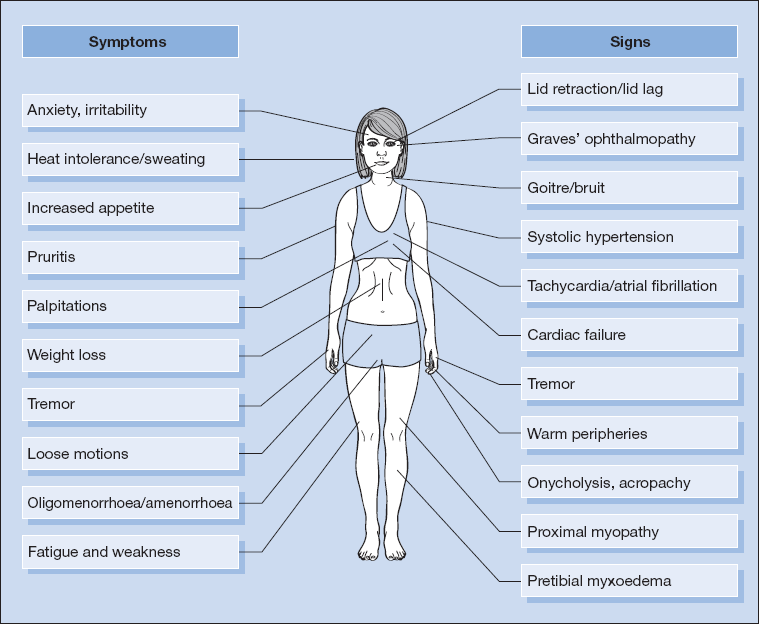
The symptoms and signs of thyrotoxicosis are shown in Fig. 16.1. Several of these are reminiscent of a ‘hyperadrenergic state’. However, other features are specific for the underlying disorder (see Box 16.1).
 Box 16.1 Specific Features of Graves’ Disease
Box 16.1 Specific Features of Graves’ Disease| System/organ | Features |
| Eyes | Exophthalmos/proptosis (may be unilateral) |
| Ophthalmoplegia (especially upward and lateral gaze) | |
| Chemosis | |
| Periorbital oedema | |
| Skin/nails | Pretibial myxoedema |
| Thyroid acropachy | |
| Thyroid | Bruit (reflecting increased vascularity of gland) |
Other Presentations
- hyperactivity, increased linear growth and weight gain in children
- apathy and depression, atrial fibrillation and cardiac failure in the elderly
- thyroid crisis – mainly seen in patients with unrecognised or poorly controlled disease; intercurrent illness, surgery or radioiodine therapy may serve as precipitants; typical features include hyperpyrexia, profuse sweating, restlessness, confusion/psychosis, dysrhythmias and cardiac failure. If untreated coma and death may ensue.
Differential Diagnosis
Thyrotoxicosis may be difficult to differentiate from an anxiety state (with hyperadrenergic features), particularly when this is coincidentally associated with a simple goitre.
Investigation
- TSH – fully suppressed (i.e. < 0.1 mU/l. NB Modern TSH assays may report even lower limits of detection, e.g. < 0.03 mU/l).
- FT4 and FT3 – typically both are elevated, although FT4 may fall within the reference range, but with raised FT3 in cases of ‘T3-toxicosis’.
- TSH receptor antibody (TRAb) – many laboratories now routinely offer TRAb measurement, which is both sensitive and specific for Graves’ disease.
NB Thyroid peroxidase (TPO) titres are not elevated in all patients with Graves’ disease and are not therefore routinely measured.
- A radioiodine or (more commonly) technetium uptake scan can help distinguish Graves’ disease, toxic nodular goitre, toxic adenoma or thyroiditis in cases where there are no specific clinical features of Graves’ disease (Box 16.1) and TRAb is not raised.
- CT/MRI of the orbits may be required to assess the extent of eye disease in Graves’ ophthalmopathy.
Treatment
β-blockers
- Preferably non-selective, e.g. propranolol 20–40 mg t.d.s. for rapid relief of symptoms.
Antithyroid Drug (ATD) Therapy
- Carbimazole (CBZ) or propylthiouracil (PTU): result in long-term remission in
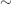 40–50% of cases of Graves’ disease; regimens include (1) ‘titration’, i.e. beginning with a higher dose (e.g. CBZ 40–60 mg/day), then titrating to a lower maintenance level (e.g. CBZ 5–15 mg/day) and continuing for 12–18 months; (2) ‘block and replace’, in which the ATD dose is maintained at a higher level (e.g. CBZ 40 mg/day), with thyroxine added back (once FT4/FT3 levels are controlled), and typically continued for 6–12 months.
40–50% of cases of Graves’ disease; regimens include (1) ‘titration’, i.e. beginning with a higher dose (e.g. CBZ 40–60 mg/day), then titrating to a lower maintenance level (e.g. CBZ 5–15 mg/day) and continuing for 12–18 months; (2) ‘block and replace’, in which the ATD dose is maintained at a higher level (e.g. CBZ 40 mg/day), with thyroxine added back (once FT4/FT3 levels are controlled), and typically continued for 6–12 months.
- ATDs control, but do not cure, thyrotoxicosis in toxic multinodular goitre/toxic solitary adenoma; ATDs are ineffective in cases of inflammatory/destructive thyroiditis where stored hormone is released – β-blockers are preferred to block peripheral actions of excess hormone.
- It is important to avoid inducing hypothyroidism, especially in patients with co-existent Graves’ ophthalmopathy, which can be exacerbated.
- Side effects of ATDs include skin rashes (often minor and respond to antihistamines or changing agent) and the more serious agranulocytosis and/or thrombocytopenia. All patients on ATDs must therefore be warned of this possibility and given instructions (including in written format) advising them to immediately stop treatment and attend their GP or local hospital for a full blood count if they develop a sore throat, mouth ulceration or fever. If confirmed, the patient should not be restarted on/switched to an alternative ATD, as the risk of recurrence is high. Hepatic dysfunction (PTU) and cholestatic jaundice (CBZ) are also important recognised side effects.
- Traditionally treatment with PTU in a titration regimen has been preferred in pregnancy because of the risk, albeit rare, of fetal malformation (in particular aplasia cutis) with CBZ therapy; however, concerns regarding potential PTU-induced hepatic dysfunction have resulted in many clinicians now favouring a mixed regimen whereby PTU is used during the first trimester, but if ongoing treatment is required thereafter, the woman is switched to CBZ.
Radioactive Iodine Therapy (RAI; 131I)
- May be used as first line therapy (especially for toxic nodular goitre/nodule) or following ATD failure.
- Typically given in doses ranging from 300–800 MBq; using higher doses reduces rates of relapse but increases rates of hypothyroidism.
- ATDs must be discontinued prior to RAI (
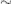 1 week before for CBZ, but longer for PTU) to ensure adequate thyroidal uptake, but can be restarted 5–7 days later and continued for 2–3 months, at which point residual thyroid status should be assessed.
1 week before for CBZ, but longer for PTU) to ensure adequate thyroidal uptake, but can be restarted 5–7 days later and continued for 2–3 months, at which point residual thyroid status should be assessed.
- Patients with a single toxic adenoma or a toxic multinodular goitre can receive a large dose of RAI with relatively little chance of subsequent hypothyroidism, because the unaffected parts of the thyroid lie dormant following suppression of TSH by excessive thyroid hormone secretion and therefore do not take up the RAI.
- RAI is contraindicated in pregnancy (which should be avoided for 6 months after treatment) and in breast-feeding mothers.
- Most centres avoid RAI in patients with severe Graves’ ophthalmopathy, but permit treatment in mild to moderate cases under steroid cover (e.g. prednisolone 30–40 mg/day).
Surgery
- Total or subtotal thyroidectomy may be performed.
- Normally reserved for those with relapsing thyrotoxicosis who decline or who are not suitable for RAI, or if there are significant compressive symptoms.
- Potential complications include haemorrhage, vocal cord paresis, hypoparathyroidism and hypothyroidism.
Treatment of Complications
Eyes
- Control of the underlying thyrotoxicosis is essential in all patients; lid retraction usually resolves with restoration of euthyroidism.
- Simple lubricants and taping the eyelids closed at night may help in milder cases.
- In severe cases of Graves’ ophthalmopathy, and in those failing to improve with correction of the thyroid disturbance, treatment with high dose corticosteroids, other immunosuppressive agents, orbital decompression or radiotherapy may be required.
- Smoking exacerbates ophthalmopathy.
Atrial Fibrillation
- Atrial fibrillation responds poorly to digoxin and larger doses are often needed until the patient is rendered euthyroid. Propranolol or other β-blockers may provide better rate control.
- Anticoagulation is also required as the risk of embolisation is relatively high.
Thyrotoxic Crisis/Thyroid Storm
This is a rare but potentially life-threatening disorder, which requires urgent treatment targeted at various steps in the thyroid hormone synthesis/action pathway:
- Stop further hormone synthesis: CBZ (20 mg orally or via nasogastric tube 4–6 hourly) or PTU (200 mg orally or via nasogastric tube 4–6 hourly).
- Impair release of stored hormone: sodium iodide (0.5–1 g IV 12 hourly) or saturated solution of potassium iodide (6–8 drops orally every 6 h); the radiographic contrast agents ipodate and iopanoic acid may also be used and have the added benefit of markedly impairing T4 to T3 conversion.
- Block peripheral manifestations of excess thyroid hormones: propranolol (initially 0.5–2 mg IV slowly, followed by 40–80 mg orally 6–8 hourly). Verapamil can be used in those with a history of asthma.
- High dose glucocorticoids: dexamethasone (2 mg orally or IV 6–8 hourly) reduces peripheral conversion of T4 to T3.
- Supportive measures: O2 therapy, intravenous fluids, active cooling, diuretics, chlorpromazine as indicated.
Hypothyroidism
Hypothyroidism is the clinical condition resulting from low levels of circulating thyroid hormones. The term ‘myxoedema’ refers to the deposition of mucopolysaccharide beneath the skin, producing a non-pitting swelling of the subcutaneous tissues.
Hypothyroidism is common, with a prevalence of 1–2% in the general population. Females are much more commonly affected (female to male ratio 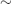 10 : 1), reflecting the high proportion of cases due to autoimmune disease (see below).
10 : 1), reflecting the high proportion of cases due to autoimmune disease (see below).
Congenital hypothyroidism occurs in 1 in 3,000 to 1 in 4,000 live births in the UK.
Aetiology
The causes of hypothyroidism are shown in Table 16.3.
Table 16.3 Causes of Hypothyroidism
| Type | Frequency | Condition(s) |
| Primary | Common | Autoimmune (e.g. Hashimoto’s thyroiditis – typically associated with a goitre: atrophic thyroiditis when the gland atrophies without producing a goitre) |
| Previous treatment for thyrotoxicosis (e.g. surgery, RAI) | ||
| Less common | Defects of hormone synthesis (e.g. iodine deficiency or excess, ATD therapy, lithium, amiodarone or, rarely, dyshormonogenesis) | |
| Thyroiditis (often transient) | ||
| Rare | Infiltration | |
| Thyroid gland hypoplasia or agenesis | ||
| Secondary | Less common | Hypothalamic or pituitary disorders |
| RAI, radioactive iodine therapy; ATD, antithyroid drug therapy. | ||
Clinical Presentation
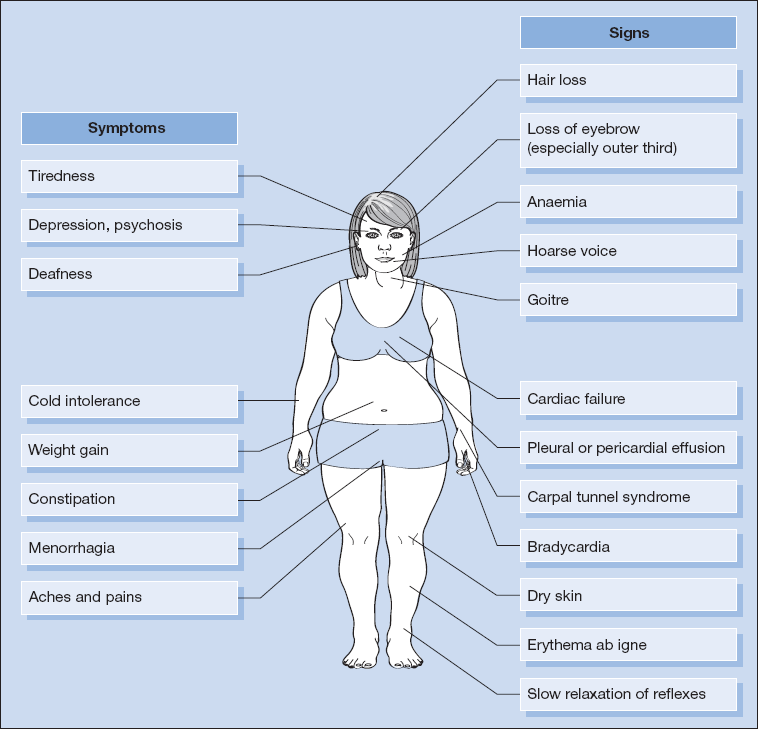
The symptoms and signs of hypothyroidism are shown in Fig. 16.2.
Other Presentations
- Subclinical hypothyroidism – characterised biochemically by normal FT4 and FT3 levels in the presence of a mildly elevated TSH. The patient may be asymptomatic or manifest mild hypothyroid features or hypercholesterolaemia.
- Pregnancy – untreated maternal hypothyroidism is associated with higher rates of miscarriage, stillbirth and congenital abnormalities. Even mild maternal hypothyroidism may be associated with a reduction in IQ of the offspring. Thyroxine requirements can increase by 50–100% during pregnancy and thyroid function tests should ideally be checked prior to conception and at regular intervals during pregnancy.
- Myxoedema coma – a rare but life-threatening medical emergency.
Investigation
- Serum FT4 is reduced and this stimulates pituitary secretion of TSH (raised in primary hypothyroidism). FT3 is also low, although routine measurement is not required in most instances.
- Elevated antithyroid peroxidase (TPO) antibodies.
- The serum cholesterol is often raised and creatine kinase (CK) may also be elevated, reflecting hepatic and muscle hypothyroidism respectively.
- Anaemia (microcytic if menorrhagia, macrocytic if co-existent pernicious anaemia, or normocytic).
- ECG shows slow rate and low voltage with flattened or inverted T waves.
NB Check for use of drugs, e.g. lithium, amiodarone. Amiodarone is rich in iodine and also inhibits peripheral conversion of T4 to T3, making thyroid investigations more difficult to interpret. Ideally, before starting treatment with amiodarone, basal FT3, FT4 and TSH levels should be checked to identify any underlying thyroid disease.
- The finding of low FT4 and FT3 levels with an inappropriately low or normal TSH is strongly suggestive of a central (hypothalamic/pituitary) disorder (p. 214).
- Low TSH with low FT3 and low/low-normal FT4 may also be seen in non-thyroidal illness (so-called sick-euthyroid syndrome).
Treatment
- Standard treatment is with levo-thyroxine (L-T4), typically beginning with a dose of 50 mcg/day.
- A full replacement dose (e.g. in an adult patient post-total thyroidectomy) is
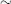 1.6 mcg/kg/day.
1.6 mcg/kg/day.
- Lower starting doses should be used in the elderly or in those with known or suspected ischaemic heart disease.
- Reassess clinically and check FT4 and TSH at 4- to 6-weekly intervals until stabilised on treatment.
- In primary hypothyroidism, titrate L-T4 to achieve TSH level in the lower half of the reference range (i.e. 0.4–2.0 mU/l); in central hypothyroidism TSH is unreliable and treatment should be titrated to alleviate symptoms and achieve an FT4 level in the normal (typically upper half of the) reference range.
NB In subjects with suspected co-existent hypoadrenalism do not start L-T4 until the diagnosis has been excluded or confirmed and glucocorticoid replacement commenced.
- Myxoedema coma: treatment includes ventilatory and circulatory support, correction of hypothermia and hypoglycaemia, glucocorticoid replacement until normal adrenal reserve is demonstrated, treatment of precipitating event and thyroid hormone replacement (L-T4 or L-T3 – dose and regimen should be decided in conjunction with an endocrinologist).
Thyroiditis
Acute Thyroiditis
- Although relatively uncommon, acute thyroiditis may follow an upper respiratory tract or other infection.
- There is fever and malaise, usually with some local swelling and tenderness of the gland and sometimes dysphagia.
- Initially the patient may experience thyrotoxic features as stored hormone is released from the gland, prior to developing hypothyroidism which may be transient or occasionally permanent.
- Classical appearance on radioiodine/technetium scan with very low or absent uptake.
- Treatment with simple analgesia (e.g. NSAID) may suffice.
- Occasionally prednisolone 30 mg/day is necessary, but this can usually be tailed off rapidly.
- ATDs are ineffective in the treatment of the thyrotoxic phase; β-blockers control symptoms.
Post-Partum Thyroiditis
- typically occurs within the first 6 months post-delivery
- usually painless
- thyrotoxic phase must be distinguished from Graves’ disease developing/relapsing in the post-partum period. TRAb antibodies are typically raised in the latter.
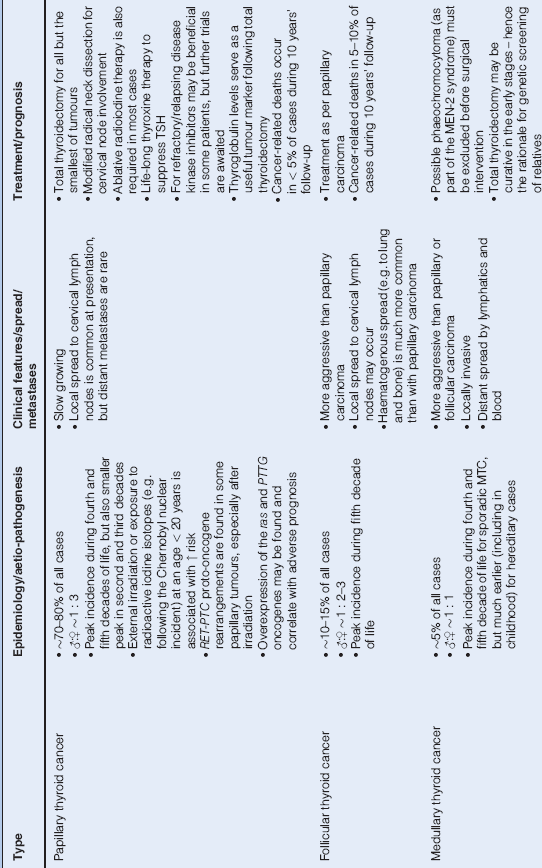
Thyroid Cancer (p. 215)
- commonest endocrine cancer, although still relatively rare
- Table 16.4 summarises important features of each of the subtypes
Table 16.4 Thyroid Malignancy

Pituitary
Pituitary Anatomy and Physiology
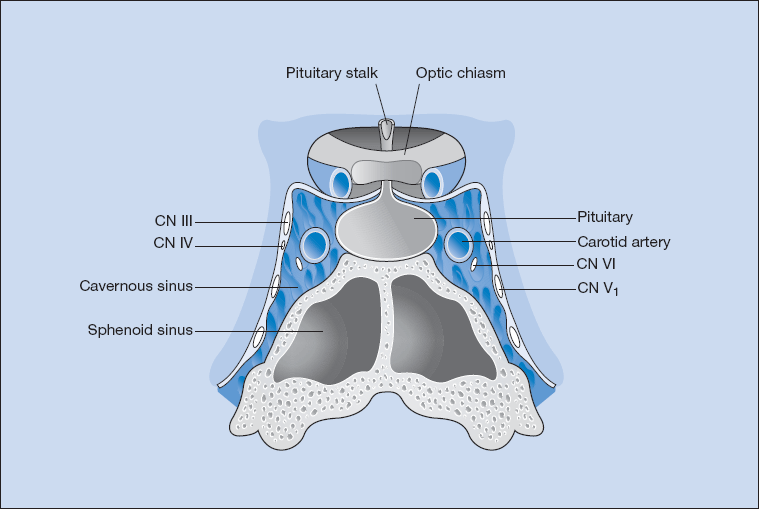
The hypothalamus and pituitary lie in close proximity to each other and are connected by the pituitary stalk. Both are surrounded by a number of important structures and the pituitary gland sits within a bony seat, the sella turcica (Fig. 16.3). The optic chiasm lies just above the pituitary fossa, and on either side are the cavernous sinuses (venous lakes) through which the intracavernous carotid artery passes. The third, fourth, upper division of the fifth and sixth cranial nerves lie within the lateral and inferior aspects of the cavernous sinuses, rendering them susceptible to compression by tumours with parasellar extension. The sphenoid sinus, which is below the pituitary fossa, is the route through which the pituitary gland is approached during transsphenoidal surgery (Fig. 16.3).
Figure 16.3 Pituitary anatomy: line drawing demonstrating the relationship of the pituitary gland to adjacent vascular and neurological structures.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree