T3, triiodothyronine; T4, thyroxine; TBG, thyroxine-binding globulin; TSH, thyroid-stimulating hormone.
Hypothyroidism
GENERAL PRINCIPLES
- Primary hypothyroidism (due to disease of the thyroid itself) accounts for >90% of cases.1
- Hypothyroidism is readily treatable and should be suspected in any patient with compatible symptoms, especially in the presence of a goiter or a history of radioactive iodine (RAI) therapy or thyroid surgery.
- Chronic lymphocytic thyroiditis (Hashimoto disease) is the most common cause and may be associated with Addison disease and other endocrine deficits. Its prevalence is greatest in women and increases with age.
- Iatrogenic hypothyroidism due to thyroidectomy and RAI therapy is also a common cause.
- Transient hypothyroidism occurs in postpartum thyroiditis and subacute thyroiditis, usually after a period of hyperthyroidism.
- Drugs that may cause hypothyroidism include iodine and iodine-containing drugs like amiodarone, lithium, α- and β-interferon, interleukin-2, thalidomide, sunitinib, and bexarotene.
- Secondary hypothyroidism due to TSH deficiency is uncommon but may occur in any disorder of the pituitary or hypothalamus. It rarely occurs without other evidence of pituitary disease.
DIAGNOSIS
Clinical Presentation
- Most symptoms of hypothyroidism are nonspecific and develop gradually. They include cold intolerance, fatigue, somnolence, poor memory, constipation, menorrhagia, myalgias, and hoarseness.
- Mild weight gain may occur, but hypothyroidism does not cause obesity.
- Signs include slow deep tendon reflex relaxation, bradycardia, facial and periorbital edema, dry skin, and nonpitting edema (myxedema).
- Rare manifestations include hypoventilation, pericardial or pleural effusions, deafness, and carpal tunnel syndrome.
Diagnostic Testing
- Laboratory findings may include hyponatremia and elevated plasma levels of cholesterol, triglycerides, and creatine kinase.
- The ECG may show low-voltage and T-wave abnormalities.
- Thyroid imaging with ultrasound or radionuclide scan is not useful in diagnosis of hypothyroidism.
- In suspected primary hypothyroidism, TSH is the best initial diagnostic test. A normal value excludes primary hypothyroidism, and a markedly elevated value (>20 μU/mL) confirms the diagnosis. If plasma TSH is elevated moderately (5 to 20 μU/mL), plasma free T4 should be measured. A low free T4 confirms clinical hypothyroidism.
- A clearly normal free T4 with an elevated plasma TSH indicates subclinical hypothyroidism, in which thyroid function is impaired but increased secretion of TSH maintains plasma T4 levels within the reference range. These patients may have nonspecific symptoms that are compatible with hypothyroidism as well as a mild increase in serum cholesterol and low-density lipoprotein cholesterol levels. They develop clinical hypothyroidism at a rate of 2.5% per year.
- If secondary hypothyroidism is suspected because of evidence of pituitary disease, plasma free T4 should be measured. Plasma TSH levels are usually within the reference range in secondary hypothyroidism and cannot be used alone to make this diagnosis. Patients with secondary hypothyroidism should be evaluated for other pituitary hormone deficits and for a mass lesion of the pituitary or hypothalamus.
- In severe nonthyroidal illness, the diagnosis of hypothyroidism may be difficult. Plasma free T4 measured by routine assays may be low. Plasma TSH is still the best initial diagnostic test. Marked elevation of plasma TSH (>20 μU/mL) establishes the diagnosis of primary hypothyroidism. A normal TSH value is strong evidence that the patient is euthyroid, except when there is evidence of pituitary or hypothalamic disease, in which case free T4 should be measured. Moderate elevations of plasma TSH (<20 μU/mL) may occur in euthyroid patients with nonthyroidal illness and are not specific for hypothyroidism.
TREATMENT
Thyroid Hormone Replacement
- Levothyroxine (T4) is the drug of choice.1 The average replacement dose is 1.6 μg/kg PO qd, and most patients require doses between 75 and 150 μg qd. Young and middle-aged patients should be started on 100 μg daily. In otherwise healthy elderly patients, the initial dose should be 50 μg daily. Patients with heart disease should be started on 25 μg daily and monitored carefully for exacerbation of cardiac symptoms.
- The need for lifelong treatment should be emphasized.
- Thyroxine should be taken 30 minutes before a meal, since dietary fiber interferes with its absorption, and should not be taken with medications such as calcium or iron supplements that affect its absorption.
Follow-Up and Dose Adjustment
- In primary hypothyroidism, the goal of therapy is to maintain plasma TSH within the normal range. After 6 to 8 weeks, plasma TSH should be measured. The dose of T4 then should be adjusted in 12- to 25-μg increments at intervals of 6 to 8 weeks until plasma TSH is normal. Thereafter, annual TSH measurement is adequate to monitor therapy.
- Overtreatment, indicated by a plasma TSH below the normal range, should be avoided, as it increases the risk of osteoporosis and atrial fibrillation.
- In secondary hypothyroidism, plasma TSH cannot be used to adjust therapy. The goal of therapy is to maintain plasma free T4 near the middle of the reference range. The dose of T4 should be adjusted at 6- to 8-week intervals until this goal is achieved. Thereafter, annual measurement of plasma free T4 is adequate to monitor therapy.
- Coronary artery disease may be exacerbated by treatment of hypothyroidism. The dose should be increased slowly, with careful attention to worsening angina, heart failure, or arrhythmias.
Difficult-to-Control Hypothyroidism
- Difficulty in controlling hypothyroidism is most often due to poor compliance with therapy. Observed therapy may be necessary in some cases.
- Other causes of increasing T4 requirements include the following:
- Malabsorption due to intestinal disease
- Drugs that interfere with T4 absorption (e.g., calcium carbonate, ferrous sulfate, colesevelam, cholestyramine, sucralfate, aluminum hydroxide)
- Other drug interactions that increase T4 clearance (e.g., rifampin, carbamazepine, phenytoin, estrogen) or block conversion of T4 to T3 (amiodarone)
- Pregnancy, in which T4 requirement increases in the first trimester
- Gradual failure of remaining endogenous thyroid function after treatment of hyperthyroidism
- Malabsorption due to intestinal disease
Subclinical Hypothyroidism
- Subclinical hypothyroidism should be treated with T4 if any of the following is present: symptoms compatible with hypothyroidism, goiter, hypercholesterolemia that warrants treatment, and plasma TSH >10 μU/mL.2 Untreated patients should be monitored annually, and T4 should be started if symptoms develop or serum TSH increases to >10 μU/mL.
Pregnancy
- Thyroxine dose increases by an average of 50% in the first half of pregnancy.3 In women with primary hypothyroidism, plasma TSH should be measured as soon as pregnancy is confirmed and monthly thereafter through the second trimester.
- The thyroxine dose should be increased as needed to maintain plasma TSH within the lower half of the normal range. After delivery, the prepregnancy dose should be resumed.
Urgent Therapy
- Urgent therapy is rarely necessary for hypothyroidism.
- Most patients with hypothyroidism and concomitant illness can be treated in the usual manner; however, hypothyroidism may impair survival in critical illness by contributing to hypoventilation, hypotension, hypothermia, bradycardia, or hyponatremia. Such patients should be admitted to the hospital for therapy of hypothyroidism and the concomitant illness.
- Confirmatory tests should be obtained before thyroid hormone therapy is started in a severely ill patient, including serum TSH and free T4.
- T4, 50 to 100 μg IV, can be given every 6 to 8 hours for 24 hours, followed by 75 to 100 μg IV daily until oral intake is possible.
- Such rapid correction is warranted only in extremely ill patients.
- Vital signs and cardiac rhythm should be monitored carefully to detect early signs of exacerbation of heart disease.
- Hydrocortisone, 50 mg IV every 8 hours, usually is recommended during rapid treatment with thyroid hormone on the grounds that replacement of thyroid hormone may precipitate adrenal failure.
Hyperthyroidism
GENERAL PRINCIPLES
- Hyperthyroidism should be suspected in any patient with compatible symptoms, as it is a readily treatable disorder that may become highly debilitating.4
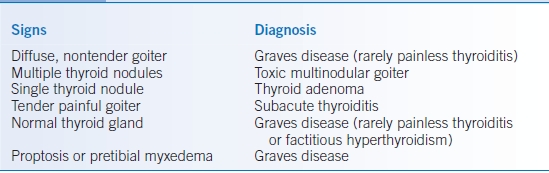
- Graves disease causes most cases of hyperthyroidism, especially in young patients. This autoimmune disorder may also cause two signs that are not found in other causes of hyperthyroidism: proptosis (exophthalmos) and pretibial myxedema.
- Toxic multinodular goiter (MNG) is a common cause in older patients.
- Unusual causes include iodine-induced hyperthyroidism, usually precipitated by drugs (e.g., amiodarone or radiographic contrast media), thyroid adenomas (which present as a single nodule), subacute thyroiditis (painful tender goiter with transient hyperthyroidism), painless thyroiditis (nontender goiter with transient hyperthyroidism, often postpartum), and surreptitious ingestion of thyroid hormone.
- TSH-induced hyperthyroidism is extremely rare.
DIAGNOSIS
Clinical Presentation
- Symptoms include heat intolerance, weight loss, weakness, palpitations, oligomenorrhea, and anxiety.
- Signs include brisk deep tendon reflexes, fine tremor, proximal weakness, stare, and eyelid lag.
- Cardiac abnormalities may be prominent, including sinus tachycardia, atrial fibrillation, and exacerbation of coronary artery disease or heart failure. In the elderly, hyperthyroidism may present with only atrial fibrillation, heart failure, weakness, or weight loss, and a high index of suspicion is needed to make the diagnosis.
- Presence of proptosis or pretibial myxedema indicates Graves disease (although many patients with Graves disease lack these signs).
- Palpation of the thyroid can determine whether a diffuse or nodular goiter is present; most hyperthyroid patients with a diffuse nontender goiter have Graves disease.
- History of recent pregnancy, neck pain, or iodine administration suggests causes other than Graves disease.
- The differential diagnosis is presented in Table 21-2.
TABLE 21-2 Differential Diagnosis of Hyperthyroidism
Diagnostic Testing
- Plasma TSH is the best initial diagnostic test.
- If plasma TSH is <0.1 μU/mL, plasma free T4 should be measured to determine the severity of hyperthyroidism and as a baseline for therapy.
- If plasma free T4 is elevated, the diagnosis of clinical hyperthyroidism is established.
- If plasma TSH is <0.1 μU/mL but free T4 is normal, the patient may have clinical hyperthyroidism due to elevation of plasma T3 alone; in this case, plasma T3 should be measured. This combination of test results may also be due to suppression of TSH by nonthyroidal illness.
- If plasma TSH is <0.1 μU/mL, plasma free T4 should be measured to determine the severity of hyperthyroidism and as a baseline for therapy.
- Subclinical hyperthyroidism may lower TSH to <0.1 μU/mL, and therefore, suppression of TSH alone does not confirm that symptoms are due to hyperthyroidism. Subclinical hyperthyroidism is present when the plasma TSH is suppressed to <0.1 μU/mL, but the patient has no symptoms that are definitely caused by hyperthyroidism and plasma levels of T4 and T3 are normal.5
- In rare cases, 24-hour RAI uptake (RAIU) is needed to distinguish Graves disease or toxic MNG (in which RAIU is elevated) from postpartum thyroiditis, iodine-induced hyperthyroidism, or factitious hyperthyroidism (in which RAIU is very low).
TREATMENT
- Some forms of hyperthyroidism (subacute or postpartum thyroiditis) are transient and require only symptomatic therapy.
- Three methods are available for definitive therapy: RAI, thionamides, and subtotal thyroidectomy, none of which controls hyperthyroidism rapidly.
- During treatment, patients are followed by clinical evaluation and measurement of plasma free T4. Plasma TSH is useless in assessing the initial response to therapy, as it remains suppressed until after the patient becomes euthyroid.
- Regardless of the therapy used, all patients with Graves disease require lifelong follow-up for recurrent hyperthyroidism or development of hypothyroidism.
Symptomatic Therapy
- β-Adrenergic antagonists are used to relieve such symptoms as palpitations, tremor, and anxiety until hyperthyroidism is controlled by definitive therapy or until transient forms of hyperthyroidism subside. The initial dose of atenolol, 25 to 50 mg daily, is adjusted to alleviate symptoms and tachycardia. β-Adrenergic antagonist therapy should be reduced gradually, then stopped as hyperthyroidism is controlled.
- Verapamil at an initial dose of 40 to 80 mg tid can be used to control tachycardia in patients with contraindications to β-adrenergic antagonists.
Thionamides
- Methimazole and propylthiouracil (PTU) inhibit thyroid hormone synthesis.4 PTU also inhibits extrathyroidal conversion of T4 to T3. These drugs have no permanent effect on thyroid function. Because of a better safety profile, methimazole should be used rather than PTU except in specific situations.
- Once thyroid hormone stores are depleted (after several weeks to months), T4 levels decrease.
- In the majority of patients with Graves disease, hyperthyroidism recurs within 6 months after therapy is stopped. Spontaneous remission of Graves disease occurs in approximately one-third of patients during thionamide therapy, and, in this minority, no other treatment may be needed. Remission is more likely to occur in mild, recent-onset hyperthyroidism and with a small goiter.
- Before starting therapy, patients must be warned of side effects and precautions. Usual starting doses are methimazole, 10 to 40 mg PO daily, or PTU, 100 to 200 mg PO tid; higher initial doses can be used in severe hyperthyroidism.
- Restoration of euthyroidism takes up to several months. Patients are evaluated at 4-week intervals with assessment of clinical findings and plasma free T4. If plasma free T4 levels do not fall after 4 to 8 weeks, the dose should be increased. Doses as high as methimazole, 60 mg PO daily, or PTU, 300 mg PO qid, may be required. Once the plasma free T4 level falls to normal, the dose is adjusted to maintain plasma free T4 within the normal range.
- No consensus exists on the optimal duration of therapy, but periods of 6 months to 2 years are used most commonly. Regardless of the duration of therapy, patients must be monitored carefully for recurrence of hyperthyroidism after the drug is stopped.
- Side effects are most likely to occur within the first few months of therapy.
- Minor side effects include rash, urticaria, fever, arthralgias, and transient leukopenia. Agranulocytosis occurs in 0.3% of patients who are treated with thionamides. Other life-threatening side effects include hepatitis, vasculitis, and drug-induced lupus erythematosus. Complications usually resolve if the drug is stopped promptly.
- Patients must be warned to stop the drug immediately if jaundice or symptoms suggestive of agranulocytosis (e.g., fever, chills, sore throat) develop and to contact their physician promptly for evaluation.
- Routine monitoring of the white blood cell count is not useful for detecting agranulocytosis, which develops suddenly.
- Methimazole has been associated with congenital abnormalities and should not be used in early pregnancy or in women attempting pregnancy.
- Minor side effects include rash, urticaria, fever, arthralgias, and transient leukopenia. Agranulocytosis occurs in 0.3% of patients who are treated with thionamides. Other life-threatening side effects include hepatitis, vasculitis, and drug-induced lupus erythematosus. Complications usually resolve if the drug is stopped promptly.
Radioactive Iodine Therapy
- A single dose permanently controls hyperthyroidism in about 90% of patients, and further doses can be given if necessary.4 Usually, 24-hour RAIU is measured and used to calculate the dose.
- A pregnancy test is done immediately before therapy in potentially fertile women, since RAI is contraindicated in pregnancy.
- Thionamides interfere with RAI therapy and should be stopped 3 days before treatment. If iodine treatment has been given, it should be stopped at least 2 weeks before RAI therapy.
- Most patients with Graves disease are treated with 10 to 12 mCi, although treatment of toxic MNG requires higher doses.
- Several months are usually needed to restore euthyroidism. Patients are evaluated at 4- to 6-week intervals, with assessment of clinical findings and plasma free T4. If thyroid function stabilizes within the normal range, the interval between follow-up visits is increased gradually to annual intervals.
- T4 therapy is started if hypothyroidism develops, indicated by a low or low-normal FT4. TSH may remain suppressed for several weeks after hypothyroidism develops and is not a reliable indicator in early hypothyroidism.
- If symptomatic hyperthyroidism persists after 6 months, RAI treatment is repeated.
- Side effects:
- Hypothyroidism occurs in most patients within the first year and continues to develop at a rate of approximately 3% per year thereafter.
- A slight rise in plasma T4 may occur in the first 2 weeks after therapy, owing to release of stored hormone. This is clinically important only in patients with severe cardiac disease, which may worsen as a result. Such patients should be treated with thionamides to restore euthyroidism and to deplete stored hormone before treatment with RAI.
- No convincing evidence has been found that RAI has a clinically important effect on the course of Graves eye disease.
- RAI does not increase the risk of malignancy.
- There is no increase in congenital abnormalities in the offspring of women who conceive after RAI therapy.
- Hypothyroidism occurs in most patients within the first year and continues to develop at a rate of approximately 3% per year thereafter.
Subtotal Thyroidectomy
- This procedure provides long-term control of hyperthyroidism in most patients.4
- Surgery may trigger a perioperative exacerbation of hyperthyroidism, and patients should be prepared for surgery by one of two methods.
- A thionamide is given until the patient is nearly euthyroid. Supersaturated potassium iodide (SSKI), 40 to 80 mg (1 to 2 drops) PO bid, is then added, and surgery is scheduled 1 to 2 weeks later. Both drugs are stopped postoperatively.
- Atenolol, 50 to 100 mg daily, and SSKI, 1 to 2 drops PO bid, are started 1 to 2 weeks before surgery is scheduled. The dose of atenolol is increased, if necessary, to reduce the resting heart rate below 90 beats/minute. Atenolol, but not SSKI, is continued for 5 to 7 days after surgery.
- A thionamide is given until the patient is nearly euthyroid. Supersaturated potassium iodide (SSKI), 40 to 80 mg (1 to 2 drops) PO bid, is then added, and surgery is scheduled 1 to 2 weeks later. Both drugs are stopped postoperatively.
- Patients should be evaluated 4 to 6 weeks after surgery, with assessment of clinical findings and plasma free T4 and TSH. If thyroid function is normal, the patient is seen at 3 and 6 months and then annually. If hypothyroidism develops, T4 therapy is started. Hyperthyroidism persists or recurs in 3% to 7% of patients.
- Complications of thyroidectomy include hypothyroidism in 30% to 50% of patients and hypoparathyroidism in 3%. Rare complications include permanent vocal cord paralysis due to recurrent laryngeal nerve injury and perioperative death. The complication rate appears to depend on the experience of the surgeon.
Choice of Definitive Therapy
- In Graves disease, RAI therapy is the treatment of choice for almost all patients. It is simple, is highly effective and causes no life-threatening complications.
- RAI cannot be used in pregnancy. PTU should be used to treat hyperthyroidism in the first trimester of pregnancy, with consideration of then changing to methimazole. Thionamides provide long-term control of hyperthyroidism in fewer than one-half of patients and carry a small risk of life-threatening side effects.
- Thyroidectomy should be used only in patients who refuse RAI therapy and who relapse or develop side effects with thionamide therapy.
Other Causes of Hyperthyroidism
- Toxic MNG and toxic adenoma should be treated with RAI (except in pregnancy).
- Transient forms of hyperthyroidism due to thyroiditis should be treated symptomatically with atenolol.
- Iodine-induced hyperthyroidism is treated with methimazole and atenolol.
- Although treatment of some patients with amiodarone-induced hyperthyroidism with glucocorticoids has been advocated, nearly all patients with amiodarone-induced hyperthyroidism respond well to methimazole.6
- Subclinical hyperthyroidism increases the risk of atrial fibrillation in the elderly and predisposes to osteoporosis in postmenopausal women and should be treated in these groups of patients.5 Asymptomatic young patients can be observed for spontaneous remission or worsening hyperthyroidism that warrants therapy.
Urgent Therapy
- Urgent therapy is warranted when hyperthyroidism exacerbates heart failure or coronary artery disease and in rare patients with severe hyperthyroidism complicated by fever and delirium. Such patients should be admitted to the hospital for therapy.
- PTU, 300 mg PO every 6 hours, should be started immediately.
- Iodide (SSKI, 1 to 2 drops PO every 12 hours) should be started 2 hours after the first dose of PTU to inhibit thyroid hormone secretion rapidly.
- Propranolol, 40 mg PO every 6 hours (or an equivalent dose of a parenteral β-adrenergic antagonist), should be given to patients with angina or myocardial infarction, and the dose should be adjusted to prevent tachycardia. Propranolol may benefit some patients with heart failure and marked tachycardia but can further impair left ventricular function. In patients with clinical heart failure, it should be given only with careful monitoring of left ventricular function.
- Plasma free T4 is measured every 4 to 6 days, and treatment with iodine is discontinued when free T4 approaches the normal range.
- RAI therapy should be scheduled 2 weeks after iodine is stopped.
Hyperthyroidism in Pregnancy
- RAI is contraindicated in pregnancy, and therefore patients should be treated with PTU, with consideration of changing to methimazole after the first trimester.3 The dose should be adjusted to maintain the plasma free T4 near the upper limit of the normal range to avoid fetal hypothyroidism. The dose required often decreases in the later stages of pregnancy.
- Atenolol, 25 to 50 mg PO daily, can be used to relieve symptoms while awaiting the effects of PTU.
- The fetus and neonate should be monitored carefully for hyperthyroidism.
Euthyroid Goiter
- The diagnosis of euthyroid goiter is based on palpation of the thyroid and on evaluation of thyroid function. If the thyroid is enlarged, the examiner should determine whether the enlargement is diffuse or multinodular or whether a single nodule is present. All three forms of euthyroid goiter are common, especially in women.
- Imaging studies, such as thyroid scans or ultrasonography, provide no useful additional information about goiters that are diffuse or multinodular by palpation and should not be performed in these patients. Furthermore, 30% to 50% of people have nonpalpable thyroid nodules that are detectable by ultrasound. These nodules rarely have any clinical importance, but their incidental discovery may lead to unnecessary diagnostic testing and treatment.7
- Almost all euthyroid diffuse goiters in the United States are due to chronic lymphocytic thyroiditis (Hashimoto thyroiditis). As Hashimoto disease may also cause hypothyroidism, plasma TSH should be measured even in patients who are clinically euthyroid. Small diffuse goiters usually are asymptomatic, and therapy is seldom required. The patient should be monitored regularly for the development of hypothyroidism.
- MNG is common in older patients, especially women. Most patients are asymptomatic and require no treatment. In a few patients, hyperthyroidism (toxic MNG) develops.
- In rare patients, the gland compresses the trachea or esophagus, causing dyspnea or dysphagia, and treatment is required. Thyroxine treatment has little or no effect on the size of MNGs and is not indicated. RAI therapy reduces gland size and relieves symptoms in most patients.8 Subtotal thyroidectomy can also be used to relieve compressive symptoms.
- The risk of malignancy in MNG is low and is comparable to the frequency of incidental thyroid carcinoma in clinically normal glands. Evaluation for thyroid carcinoma with needle biopsy is warranted only if one nodule is disproportionately enlarged.
- In rare patients, the gland compresses the trachea or esophagus, causing dyspnea or dysphagia, and treatment is required. Thyroxine treatment has little or no effect on the size of MNGs and is not indicated. RAI therapy reduces gland size and relieves symptoms in most patients.8 Subtotal thyroidectomy can also be used to relieve compressive symptoms.
Single Thyroid Nodules
- Single thyroid nodules are usually benign, but about 5% are thyroid carcinomas.9
- Clinical findings that increase the likelihood of carcinoma include the presence of cervical lymphadenopathy, a history of radiation to the head or neck in childhood, and a family history of medullary thyroid carcinoma or multiple endocrine neoplasia syndromes type 2A or 2B. A hard fixed nodule, recent nodule growth, or hoarseness due to vocal cord paralysis also suggests malignancy. However, most patients with thyroid carcinomas have none of these risk factors, and nearly all palpable single thyroid nodules should be evaluated with needle aspiration biopsy. Patients with thyroid carcinoma should be managed in consultation with an endocrinologist.
- Nodules with benign cytology should be reevaluated periodically by palpation and biopsied again if they enlarge.
- T4 therapy has little or no effect on the size of single thyroid nodules and is not indicated.
- Imaging studies cannot distinguish benign from malignant nodules and are not necessary for the evaluation of a palpable thyroid nodule.
- The management of nonpalpable thyroid nodules discovered incidentally by ultrasound is controversial.7
Adrenal Failure
GENERAL PRINCIPLES
- Adrenal failure may be due to disease of the adrenal glands (primary adrenal failure, Addison disease), with deficiency of cortisol and aldosterone and elevated plasma adrenocorticotropic hormone (ACTH) or due to ACTH deficiency caused by disorders of the pituitary or hypothalamus (secondary adrenal failure) with deficiency of cortisol alone.
- Findings in adrenal failure are nonspecific and, without a high index of suspicion, the diagnosis of this potentially lethal but readily treatable disease is easily missed.
- Adrenal failure should be suspected in patients with hypotension (including orthostatic hypotension), persistent nausea, weight loss, hyponatremia, or hyperkalemia.
- Primary adrenal failure:
- Often due to autoimmune adrenalitis, which may be associated with other endocrine deficits (e.g., hypothyroidism).
- Infections of the adrenal gland such as tuberculosis and histoplasmosis may cause adrenal failure.
- Hemorrhagic adrenal infarction may occur in the postoperative period, in coagulation disorders and hypercoagulable states, and in sepsis (i.e., Waterhouse-Friderichsen syndrome). Adrenal hemorrhage often causes abdominal or flank pain and fever; CT scan of the abdomen reveals high-density bilateral adrenal masses.
- Adrenoleukodystrophy causes adrenal failure in young males.
- In patients with AIDS, adrenal failure may develop because of disseminated cytomegalovirus, mycobacterial or fungal infection, or adrenal lymphoma, or treatment with ketoconazole, which inhibits steroid hormone synthesis.
- Often due to autoimmune adrenalitis, which may be associated with other endocrine deficits (e.g., hypothyroidism).
- Secondary adrenal failure is most often due to glucocorticoid therapy; ACTH suppression may persist for a year after therapy is stopped. Any disorder of the pituitary or hypothalamus can cause ACTH deficiency, but usually other evidence of these disorders can be seen.
DIAGNOSIS
Clinical Presentation
- Symptoms include anorexia, nausea, vomiting, weight loss, weakness, and fatigue.
- Orthostatic hypotension and hyponatremia are common.
- Usually symptoms are chronic, but shock that is fatal unless treated promptly may develop suddenly. Often, this adrenal crisis is triggered by illness, injury, or surgery.
- Hyperpigmentation (due to marked ACTH excess), hyperkalemia, and volume depletion (due to aldosterone deficiency) occur only in primary adrenal failure.
Diagnostic Testing
- The short cosyntropin stimulation test is used for diagnosis.
- Cosyntropin, 250 μg, is given IV or IM, and plasma cortisol is measured 30 minutes later. The normal response is a stimulated plasma cortisol >20 μg/dL.
- This test detects primary and secondary adrenal failure, except within a few weeks of onset of pituitary dysfunction (e.g., shortly after pituitary surgery).
- Cosyntropin, 250 μg, is given IV or IM, and plasma cortisol is measured 30 minutes later. The normal response is a stimulated plasma cortisol >20 μg/dL.
- The distinction between primary and secondary adrenal failure usually is clear.
- Hyperkalemia, hyperpigmentation, or other autoimmune endocrine deficits indicate primary adrenal failure, whereas deficits of other pituitary hormones, symptoms of a pituitary mass (e.g., headache, visual field loss), or known pituitary or hypothalamic disease indicate secondary adrenal failure.
- If the cause is unclear, the plasma ACTH level distinguishes primary adrenal failure (in which it is markedly elevated) from secondary adrenal failure.
- Evidence of adrenal enlargement or calcification on abdominal CT indicates that the cause is infection or hemorrhage.
- Patients with secondary adrenal failure should be tested for other pituitary hormone deficiencies and should be evaluated for a pituitary or hypothalamic tumor.
- Hyperkalemia, hyperpigmentation, or other autoimmune endocrine deficits indicate primary adrenal failure, whereas deficits of other pituitary hormones, symptoms of a pituitary mass (e.g., headache, visual field loss), or known pituitary or hypothalamic disease indicate secondary adrenal failure.
TREATMENT
Adrenal Crisis
- Adrenal crisis with hypotension must be treated immediately. These patients should be admitted to the hospital for therapy and be evaluated for an underlying illness that precipitated the crisis.
- If the diagnosis of adrenal failure is known, hydrocortisone, 100 mg IV every 8 hours, should be given, and 0.9% saline with 5% dextrose should be infused rapidly until hypotension is corrected.
- The dose of hydrocortisone is decreased gradually over several days as symptoms and precipitating illness resolve and then is changed to oral maintenance therapy.
- Mineralocorticoid replacement is not needed until the dose of hydrocortisone is <100 mg/day.
- The dose of hydrocortisone is decreased gradually over several days as symptoms and precipitating illness resolve and then is changed to oral maintenance therapy.
- If the diagnosis of adrenal failure has not been established, a single dose of dexamethasone, 10 mg IV, should be given, and a rapid infusion of 0.9% saline with 5% dextrose should be started.
- A cosyntropin stimulation test should be performed.
- Dexamethasone is used because it does not interfere with subsequent measurements of cortisol.
- After the 30-minute plasma cortisol measurement, hydrocortisone, 100 mg IV every 8 hours, should be given until the test result is known.
- A cosyntropin stimulation test should be performed.
Outpatient Maintenance Therapy
- All patients with adrenal failure require cortisol replacement with prednisone. Most patients with primary adrenal failure also require replacement of aldosterone with fludrocortisone.
- Prednisone, 5 mg PO every morning, should be started.
- Patients should initially be evaluated every 1 to 2 months.
- The dose of prednisone is adjusted to eliminate symptoms and signs of cortisol deficiency or excess, with most patients requiring between 4 mg every morning to as much as 5 mg every morning and 2.5 mg every evening.
- The goal of therapy is the lowest dose of prednisone that relieves symptoms, to avoid the possibility of producing signs of Cushing syndrome.
- Eventually, annual follow-up is adequate unless an acute illness develops.
- Concomitant therapy with rifampin, phenytoin, or phenobarbital accelerates glucocorticoid metabolism and increases the dose requirement.
- Patients should initially be evaluated every 1 to 2 months.
- During illness, injury, or the perioperative period, the dose of glucocorticoid must be increased.
- For minor illnesses, the patient should double the dose of prednisone for 3 days. If the illness resolves, maintenance dose is resumed. Vomiting requires immediate medical attention, with IV glucocorticoid therapy and IV fluid. Patients can be given a 4-mg vial of dexamethasone, to be self-administered IM for vomiting or severe illness if medical care is not immediately available.
- For severe illness or injury, hydrocortisone, 50 mg IV every 8 hours, should be given, with the dose tapered as severity of illness wanes. The same regimen is used in patients who are undergoing surgery, with the first dose of hydrocortisone given preoperatively. Usually, the dose can be reduced to maintenance therapy 2 to 3 days after uncomplicated surgery.
- For minor illnesses, the patient should double the dose of prednisone for 3 days. If the illness resolves, maintenance dose is resumed. Vomiting requires immediate medical attention, with IV glucocorticoid therapy and IV fluid. Patients can be given a 4-mg vial of dexamethasone, to be self-administered IM for vomiting or severe illness if medical care is not immediately available.
- In primary adrenal failure, fludrocortisone, 0.1 mg PO qd, should be given. During follow-up visits, supine and standing blood pressure and serum potassium should be monitored.
- The dose of fludrocortisone is adjusted to maintain blood pressure and serum potassium within the normal range; the usual dose is 0.05 to 0.2 mg PO qd.
- Patients should be educated in management of their disease, including adjustment of prednisone dose during illness. They should wear a medical identification tag or bracelet.
Cushing Syndrome
GENERAL PRINCIPLES
- Cushing syndrome (the clinical effects of increased glucocorticoid hormone) is most often iatrogenic due to therapy with glucocorticoid drugs.
- ACTH-secreting pituitary microadenomas (Cushing disease) account for approximately 80% of cases of endogenous Cushing syndrome. Adrenal tumors and ectopic ACTH secretion account for the remainder.
DIAGNOSIS
Clinical Presentation
- Clinical features include truncal obesity, rounded face, fat deposits in the supraclavicular fossae and over the posterior neck, hypertension, hirsutism, amenorrhea, and depression.
- More specific findings include thin skin, easy bruising, reddish striae, proximal muscle weakness, and osteoporosis.
- Diabetes mellitus develops in some patients.
- Hyperpigmentation or hypokalemic alkalosis suggests Cushing syndrome because of ectopic ACTH secretion.
Diagnostic Testing
- The diagnosis is based on increased cortisol excretion, lack of normal feedback inhibition of ACTH and cortisol secretion, or loss of the normal diurnal rhythm of cortisol secretion.10
- Overnight dexamethasone suppression test can be done as a screening test. 1-mg dexamethasone given PO at 11:00 pm; plasma cortisol measured at 8:00 am the next day; normal plasma cortisol level <2 μg/dL. Salivary cortisol may be measured at home during the nadir of normal plasma cortisol at 11:00 pm.
- If the overnight dexamethasone suppression test or 11 pm salivary cortisol is abnormal, 24-hour urine cortisol should be measured. Twenty-four–hour urine cortisol measurement can also be done as a screening test. A normal value virtually excludes the diagnosis.
- If the 24-hour urine cortisol is more than four times the upper limit of the reference range in a patient with compatible symptoms, the diagnosis of Cushing syndrome is established.
- In patients with milder elevations of urine cortisol, a low-dose dexamethasone suppression test should be performed. Dexamethasone, 0.5 mg PO every 6 hours, is given for 48 hours, starting at 8:00 am. Urine cortisol is measured during the last 24 hours, and plasma cortisol is measured 6 hours after the last dose of dexamethasone. Failure to suppress plasma cortisol to <2 μg/dL and urine cortisol to less than the normal reference range is diagnostic of Cushing syndrome.
- Testing should not be done during severe illness or depression, which may cause false-positive results.
- Phenytoin therapy also causes false-positive dexamethasone suppression test results by accelerating metabolism of dexamethasone.
- Random plasma cortisol levels are not useful for diagnosis, because the wide range of normal values overlaps that of Cushing syndrome.
- After the diagnosis of Cushing syndrome is made, tests to determine the cause should be done in consultation with an endocrinologist.
TREATMENT
The treatment of hypercortisolism is dependent on its cause, and a complete discussion of management is beyond the scope of this chapter. Stopping exogenous glucocorticoids when possible is clearly indicated. Other treatments usually require the assistance of an endocrinologist or neurosurgeon.
Incidental Adrenal Nodules
GENERAL PRINCIPLES
Adrenal nodules are a common incidental finding on abdominal imaging studies. Most incidentally discovered nodules are benign adrenocortical tumors that do not secrete excess hormone, but the differential diagnosis includes adrenal adenomas that cause Cushing syndrome or primary hyperaldosteronism, pheochromocytoma, adrenocortical carcinoma, and metastatic cancer.11
DIAGNOSIS
Clinical Presentation
The patient should be evaluated for symptoms and signs of Cushing syndrome. Hypertension suggests the possibility of primary hyperaldosteronism or pheochromocytoma. Episodes of headache, palpitations, and sweating suggest pheochromocytoma. Hirsutism suggests the possibility of an adrenocortical carcinoma.
Diagnostic Testing
- The imaging characteristics of the nodule may suggest a diagnosis (e.g., benign adrenocortical nodule) but are not specific enough to obviate further evaluation.
- Patients who have potentially resectable cancer elsewhere and in whom an adrenal metastasis must be excluded may require positron emission tomography.
- In other patients, the diagnostic issue is whether a syndrome of hormone excess or an adrenocortical carcinoma is present.
- Plasma potassium, fractionated metanephrines, and dehydroepiandrosterone sulfate should be measured, and an overnight dexamethasone suppression test should be performed.
- Patients with hypertension and hypokalemia should be evaluated for primary hyperaldosteronism by measuring the ratio of plasma aldosterone (in ng/dL) to plasma renin activity (in ng/mL/hour) in a single blood sample.
- This sample can be obtained from an ambulatory patient without special preparation.
- If the ratio is <20, the diagnosis of primary hyperaldosteronism is excluded, whereas a ratio of >50 makes the diagnosis very likely.
- Patients with an intermediate ratio should be further evaluated in consultation with an endocrinologist.
- This sample can be obtained from an ambulatory patient without special preparation.
- An abnormal overnight dexamethasone suppression test should be evaluated further (see Cushing Syndrome).
- Elevation of plasma dehydroepiandrosterone sulfate or a large nodule suggests adrenocortical carcinoma.
TREATMENT
- If there is clinical or biochemical evidence of a pheochromocytoma, the nodule should be resected after appropriate α-adrenergic blockade with phenoxybenzamine.
- Most incidental nodules are <4 cm in diameter, do not produce excess hormone, and do not require therapy. One repeat imaging procedure 3 to 6 months later is recommended to ensure that the nodule is not enlarging rapidly (which would suggest an adrenal carcinoma).
- A policy of resecting all nodules >4 cm in diameter appropriately treats the great majority of adrenal carcinomas while minimizing the number of benign nodules removed unnecessarily.
Hypercalcemia
GENERAL PRINCIPLES
- Approximately 50% of serum calcium is ionized (free), and the remainder is complexed, primarily to albumin. Changes in serum albumin alter total calcium concentration without affecting the clinically relevant ionized calcium level, and if serum albumin is abnormal, clinical decisions should be based on albumin-corrected or ionized calcium levels.
- Parathyroid hormone (PTH) increases serum calcium by stimulating bone resorption, increasing renal calcium reabsorption, and promoting renal conversion of vitamin D to its active metabolite calcitriol (1,25-dihydroxyvitamin D [1,25(OH)2D]). Serum calcium regulates PTH secretion by a negative feedback mechanism; hypercalcemia suppresses PTH release.
- Vitamin D is converted by the liver to 25-hydroxyvitamin D [25(OH)D], which in turn is converted by the kidney to 1,25(OH)2D. The latter metabolite increases serum calcium by promoting intestinal calcium absorption and plays a role in bone formation and resorption.
- Other factors that raise serum calcium include PTH-related peptide, which acts on PTH receptors, and some cytokines produced by plasma cells and lymphocytes.
- The major causes of hypercalcemia are listed in Table 21-3. >95% of cases are due to primary hyperparathyroidism or malignancy.
- Primary hyperparathyroidism:
- Causes most cases of mild hypercalcemia in ambulatory patients.
- It is a common disorder, especially in elderly women. Approximately 85% of cases are due to an adenoma of a single gland, 15% to enlargement of all four glands, and 1% to parathyroid carcinoma.
- Familial syndromes that include primary hyperparathyroidism (e.g., the multiple endocrine neoplasia syndromes) cause enlargement of all four glands.
- Causes most cases of mild hypercalcemia in ambulatory patients.
- Malignancy causes most severe, symptomatic hypercalcemia. Common causes of malignant hypercalcemia include the following:
- Breast carcinoma (which is usually metastatic to bone when hypercalcemia occurs).
- Squamous carcinoma of the lung, head and neck, or esophagus (which may produce humoral hypercalcemia without extensive bone metastases).
- Multiple myeloma.
- Most malignant hypercalcemia is due to secretion of PTH-related peptide by the tumor, except for myeloma, in which hypercalcemia is mediated by cytokines.
- Breast carcinoma (which is usually metastatic to bone when hypercalcemia occurs).
- Other causes of hypercalcemia are uncommon and are almost always suggested by the history or physical examination.12
- Thiazide diuretics cause persistent hypercalcemia only in patients with increased bone turnover, for example, due to mild primary hyperparathyroidism.
- Sarcoidosis and other granulomatous disorders may cause hypercalcemia by excessive synthesis of 1,25(OH)2D.
- Familial benign hypercalciuric hypercalcemia is a rare autosomal dominant disorder that causes asymptomatic hypercalcemia from birth. It is due to a genetic defect in the calcium-sensing receptor on parathyroid cells and should be suspected if there is a family history of asymptomatic hypercalcemia.
- Thiazide diuretics cause persistent hypercalcemia only in patients with increased bone turnover, for example, due to mild primary hyperparathyroidism.
TABLE 21-3 Major Causes of Hypercalcemia

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


