The benefit of medical evaluation prior to engaging in an exercise regimen is unclear. The data are not strong enough to reach consensus on identity of which groups would benefit from exercise evaluation. However, for children and adolescence, many medical bodies have guidelines for and recommend pre-participation physicals.
An initial aerobic regimen involves an “exercise prescription” of walking >30 minutes per day, 5 to 7 days per week. This amount can lead to benefits in prevention of weight gain and mortality reduction. From that point, the patient can branch and scale their exercise regimen on the basis of personal preference, fitness level, and cardiac/musculoskeletal conditions. The emphasis should be on the patient choosing an exercise regimen that they will continue indefinitely.
Pharmacotherapy
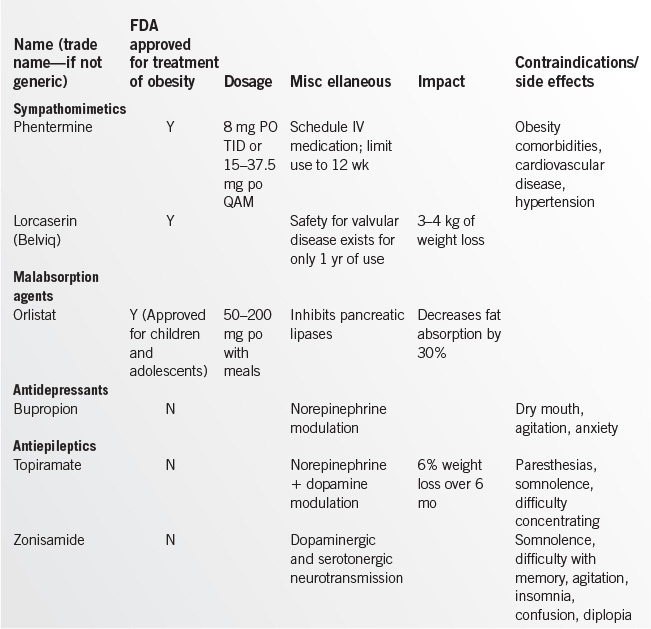
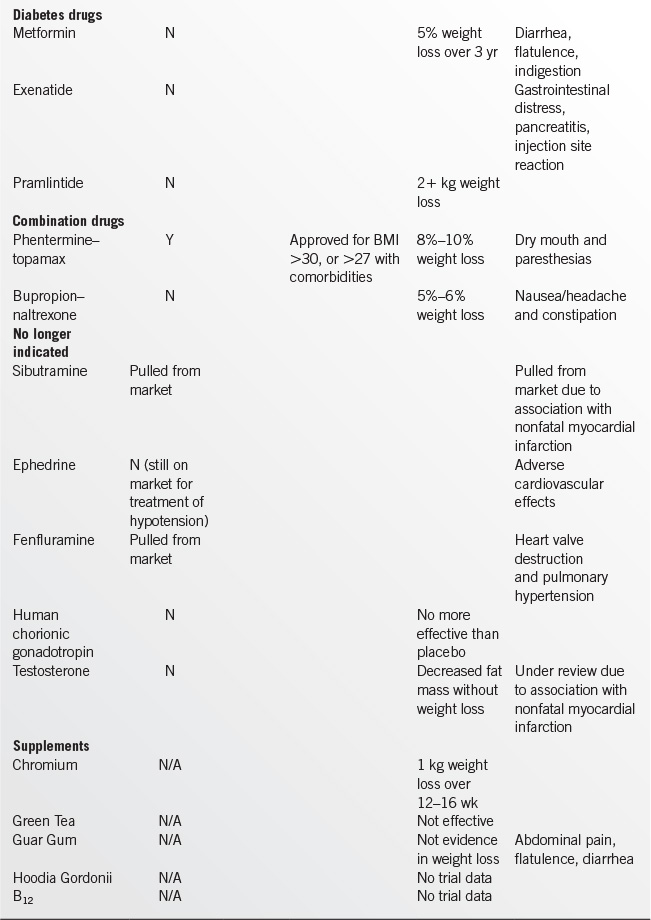
In selected patients who have failed to achieve adequate weight loss, medication is an appropriate adjunct to a low-calorie diet, physical activity, and behavior modification. Weight loss medications create an energy deficit through reduced food consumption or reduced absorption.
In considering pharmacotherapy, a provider should base their choice of medication on positive effects beyond that for weight loss. For instance, a provider may wish to consider diet and exercise with metformin or exenatide in a patient with diabetes (as opposed to a sulfonylurea), or bupropion in a depressed patient. See Table 17.1-4 for a comparison of weight loss medications.
Surgical Intervention
Consultation to a bariatric surgeon should be considered in patients with a BMI greater than 40, or BMI greater than 35 with serious medical comorbidities, who have not been successful with diet, exercise, and medical management. Bariatric surgery can be considered in children/adolescents if they have a BMI >35 kg per m2 with comorbidities or BMI >40 kg per m2, if they have reached 95% of their predicted adult stature by bone age or Tanner Stage IV, and if they have a history of failure of intensive diet/exercise programs.25
The three surgical procedures currently in use are:
• The Roux-en-Y gastric bypass creates a small stomach pouch and allows food to bypass some of the small intestine.
• Gastroplasty decreases the overall size of the stomach to create a feeling of fullness after eating a small amount of food leading to decreased caloric intake and weight loss.
• Lap-banding gastroplasty places a plastic band around the stomach that is connected to a subcutaneous pouch. This pouch is inflated with saline to apply a variable level of constriction to the stomach.
Bariatric surgery results in greater weight loss than conventional weight loss programs.26 Likewise, bariatric surgery is highly effective in treating diabetes, hyperlipidemia, and HTN27—preventing up to 40% of disease-related mortality.28
Postoperative complications of bariatric surgery include infection, anemia, vitamin B deficiencies, inadequate weight loss, and gallstone formation due to rapid weight loss. The family physician should ensure that dietary changes, physical activity, and behavior modification are a part of the overall strategy for weight reduction to ensure maintenance of weight loss over time. Cold intolerance, hair loss, and fatigue are common but tend to diminish rapidly as weight loss stabilizes.
Quarterly assessment of nutritional status and supplementation needs, food intolerances, and symptoms should occur for the first year after bariatric surgery. A variety of micronutrient deficiencies have been identified because of decreased capacity for food intake. Vitamin supplementation will be required throughout the patient’s lifetime, and annual metabolic and nutritional monitoring is recommended, although no standard exists. Vitamin and mineral deficiencies, such as decreased iron, B12, vitamin D3, and calcium, are common and may manifest many years following bariatric surgery.
OBESITY MANAGEMENT IN SPECIAL POPULATIONS
Absolute contraindications to weight loss are terminal illness and anorexia nervosa. Temporary contraindications include pregnancy and lactation; unstable psychiatric, medical, or surgical status; and bulimia nervosa. Patients with osteopenia or osteoporosis should undergo discussion of risk and undertake medical management to maintain bone mineral density.
Elderly persons are at increased risk of becoming overweight with loss of physical activity and decreased energy expenditure. Regular, moderate exercise and a diet low in fat and high in fiber can control weight while providing for nutritional needs. Issues of polypharmacy, drug interactions, and age-related physiologic factors should be considered before medication is prescribed for obese elderly patients.
ACKNOWLEDGMENT
The author wishes to acknowledge Meg Hayes for her work on the previous version of this chapter.
REFERENCES
1. Ogden CL, Carroll MD, Kit BK, et al. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA 2014;311:806.
2. Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014;384(9945):766–781.
3. Baskin ML, Ard J, Franklin F, et al. Prevalence of obesity in the United States. Obesity Rev 2005;6(1):5.
4. Calle EE, Thun MJ, Petrelli JM, et al. Body mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med 1999;341:1097–1105.
5. http://www.ncbi.nlm.nih.gov/books/NBK44660/pdf/TOC.pdf. Accessed June 2014.
6. Hart DJ, Spector TD. The relationship of obesity, fat distribution and osteoarthritis in women in the general population: the Chingford Study. J Rheumatol 1993;20:331.
7. Young T, Skatrud J, Peppard PE. Risk factors for obstructive sleep apnea in adults. JAMA 2004;291:2013.
8. Choi HK, Atkinson K, Karlson EW, et al. Obesity, weight change, hypertension, diuretic use, and risk of gout in men: the health professionals follow-up study. Arch Intern Med 2005;165:742.
9. Singh-Manoux A, Czernichow S, Elbaz A, et al. Obesity phenotypes in midlife and cognition in early old age: the Whitehall II cohort study. Neurology 2012;79:755.
10. Banim PJ, Luben RN, Bulluck H, et al. The aetiology of symptomatic gallstones quantification of the effects of obesity, alcohol and serum lipids on risk. Epidemiological and biomarker data from a UK prospective cohort study (EPIC-Norfolk). Eur J Gastroenterol Hepatol 2011;23:733.
11. Subak LL, Richter HE, Hunskaar S. Obesity and urinary incontinence: epidemiology and clinical research update. J Urol 2009;182:S2.
12. Falagas ME, Kompoti M. Obesity and infection. Lancet Infect Dis 2006;6:438.
13. Kwong JC, Campitelli MA, Rosella LC. Obesity and respiratory hospitalizations during influenza seasons in Ontario, Canada: a cohort study. Clin Infect Dis 2011;53:413.
14. Taylor EN, Stampfer MJ, Curhan GC. Obesity, weight gain, and the risk of kidney stones. JAMA 2005;293:455.
15. Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 2003;348:1625.
16. Barlow SE; Expert Committee. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics 2007;120 (Suppl 4):S164.
17. Rao G. Office-based strategies for the management of obesity. Am Fam Physician 2010; 81(12):1449–1455.
18. Allen G, Safranek S. FPIN’s clinical inquiries. Secondary causes of obesity. Am Fam Physician 2011;83(8):972–973.
19. U.S. Preventive Services Task Force. Guide to clinical preventive services. 2nd ed. Washington, DC: Office of Disease Prevention and Health Promotion; 1996.
20. Moyer VA; U.S. Preventive Services Task Force. Behavioral counseling interventions to promote a healthful diet and physical activity for cardiovascular disease prevention in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2012;157:367.
21. Slentz CA, Duscha BD, Johnson JL, et al. Effects of the amount of exercise on body weight, body composition, and measures of central obesity: STRRIDE—a randomized controlled study. Arch Intern Med 2004;164:31.
22. Lee L, Kumar S, Leong LC. The impact of five-month basic military training on the body weight and body fat of 197 moderately to severely obese Singaporean males aged 17 to 19 years. Int J Obes Relat Metab Disord 1994;18:105.
23. Avenell A, Brown TJ, McGee MA, et al. What interventions should we add to weight reducing diets in adults with obesity? A systematic review of randomized controlled trials of adding drug therapy, exercise, behaviour therapy or combinations of these interventions. J Hum Nutr Diet 2004;17:293.
24. Kokkinos P. Physical Activity, Health Benefits, and Mortality Risk. ISRN Cardiol 2012;2012;718789.
25. Pratt JS, Lenders CM, Dionne EA, et al. Best practice updates for pediatric/adolescent weight loss surgery. Obesity (Silver Spring) 2009;17:901.
26. Colquitt JL, Picot J, Loveman E, et al. Surgery for obesity. Cochrane Database Syst Rev 2009;(2):CD003641.
27. Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and metaanalysis. Am J Med 2009;122(3):248–256.
28. Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med 2007;357(8):753–761.
|
CLASSIFICATION
• Type 1 (insulin-dependent) diabetes mellitus (DM) is characterized by insulin deficiency due to autoimmune pancreatic β-cell destruction.1
• Type 2 (non–insulin-dependent) DM is characterized by insulin resistance and variable insulin secretory defects. Abdominal obesity, hypertension, and dyslipidemia often coexist as the metabolic syndrome.1
• Latent autoimmune diabetes of adulthood (LADA)
• Gestational DM (see Chapter 14.6)
• Secondary DM (not covered in this chapter)
INITIAL APPROACH TO THE PATIENT
• Clinical history yields important clues to the presence and correct classification of DM.
• Type 1 DM
![]() Recent onset of polydipsia, polyuria, significant weight loss, fatigue, and ketonuria occurs in a patient generally younger than 30 years of age.
Recent onset of polydipsia, polyuria, significant weight loss, fatigue, and ketonuria occurs in a patient generally younger than 30 years of age.
![]() Clinical duration of symptoms is relatively short despite a long prodrome of autoimmune pancreatic islet destruction.
Clinical duration of symptoms is relatively short despite a long prodrome of autoimmune pancreatic islet destruction.
• Type 2 DM
![]() Patient may present with polydipsia, polyuria, history of weight gain or loss, fatigue, glycosuria, obesity (especially abdominal), hypertension, dyslipidemia, positive family history of DM, or previous gestational DM. Traditionally, type 2 DM presented in patients generally older than 40 years of age; however, with increasing obesity, the age of onset can be in childhood.
Patient may present with polydipsia, polyuria, history of weight gain or loss, fatigue, glycosuria, obesity (especially abdominal), hypertension, dyslipidemia, positive family history of DM, or previous gestational DM. Traditionally, type 2 DM presented in patients generally older than 40 years of age; however, with increasing obesity, the age of onset can be in childhood.
![]() Clinical duration of mild hyperglycemia with minimal symptoms may be prolonged in such a way that patients may present with DM complications (peripheral neuropathy, retinopathy, nephropathy).
Clinical duration of mild hyperglycemia with minimal symptoms may be prolonged in such a way that patients may present with DM complications (peripheral neuropathy, retinopathy, nephropathy).
![]() LADA. Antibody positive DM of a more indolent nature. Many individuals can be controlled for years on medications other than insulin. Eventually, patients require full insulin replacement akin to an individual with type 1 DM.
LADA. Antibody positive DM of a more indolent nature. Many individuals can be controlled for years on medications other than insulin. Eventually, patients require full insulin replacement akin to an individual with type 1 DM.
• Gestational DM (see Chapter 14.6).
• Secondary DM. Consider DM in the context of the primary condition such as chronic pancreatitis, post-pancreatectomy, Cushing syndrome, acromegaly, cystic fibrosis, coadministration of antipsychotic medications, etc.
• Physical examination
• The presence of DM complications such as acanthosis nigricans noted on a careful initial physical examination at time of diagnosis strongly favors a diagnosis of type 2 DM.
• Laboratory diagnosis1
• Fasting serum glucose ≥126 mg per dL on two occasions in ambulatory setting.
• Casual serum glucose ≥200 mg per dL on two occasions in ambulatory setting.
• An HbA1c ≥6.5% is diagnostic for DM due to better standardization of HbA1c assays.2,3
• A 2-hour postload plasma glucose. (Glucose tolerance test is no longer routinely needed to diagnose DM.)
![]() Indications for a 2-hour postload plasma glucose test
Indications for a 2-hour postload plasma glucose test
– Equivocal serum glucose levels, especially in the presence of other stigmata of metabolic syndrome.
– Presence of complications, most commonly unexplained peripheral neuropathy, suggestive of DM when casual serum glucose and fasting serum glucose are non-diagnostic.
![]() Diagnostic when 2-hour postload plasma glucose ≥200 mg per dL
Diagnostic when 2-hour postload plasma glucose ≥200 mg per dL
![]() Other abnormalities of glucose tolerance testing
Other abnormalities of glucose tolerance testing
– Impaired glucose tolerance (IGT)
• Fasting plasma glucose <126 mg per dL
• 2-Hour postload (75 g anhydrous glucose) plasma glucose of ≥140 mg per dL and ≤199 mg per dL
– Isolated impaired fasting glucose (IFG)
• Fasting plasma glucose ≥100 mg per dL and <126 mg per dL
– Combined IGT and IFG
– Caveat: Patients with isolated IFG are at lower risk to progress to frank DM and develop complications of DM, including increased atherosclerotic complications.
• Laboratory classification. Clinical history, physical examination, ambient glucose levels, and degree of ketosis usually suffice for appropriate diagnostic classification. In equivocal settings, measurement of C-peptide or insulin levels (low in type 1 patients) coupled with glutamic acid decarboxylase antibodies, insulin autoantibodies, and pancreatic islet cell antibodies (positive in new-onset type 1 DM patients and LADA patients) allows correct classification.
• Treatment goals. Since publication of the Diabetes Control and Complications Trial (DCCT),4 the Epidemiology of Diabetic Complications Trial (EDIC) (the extension of the DCCT),5 the United Kingdom Prospective Diabetes Study (UKPDS),6 the UKPDS follow-up study,7 and position/consensus statements from the American Diabetes Association8 and the American Association of Clinical Endocrinologists,9 the ultimate goal for all patients, with few exceptions, is normalization or near-normalization of blood glucose levels within the constraints of hypoglycemia. Exceptions may include extremes of age, limited life expectancy, and advanced diabetic complications, including cardiovascular and cerebrovascular disease. Intensive glucose lowering in patients with long-standing type 2 DM (median duration 10 years) and established coronary artery disease (CAD) (35% of patients) or at high risk for CAD resulted in increased mortality and no decrease in major cardiovascular events in the ACCORD trial.10 Treatment goals will likely change on the basis of outcomes from new research; but regardless, patient training and education by a skilled team is vital to the safety and efficacy of the treatment plan.
• Short-term goals are (a) correction of hyperglycemia and ketosis, (b) elimination of hypoglycemia, and (c) reintegration of patient into society.
• Long-term goals include preservation of residual insulin production in type 1 DM patients through early physiologic insulin replacement, facilitating long-term optimal glycemic control, and forestalling “brittleness.” The key is attainment and maintenance of normal or near-normal body weight through diet and exercise to optimize insulin sensitivity, minimize insulin requirements, and minimize cardiovascular risk. Prevention of microvascular and macrovascular complications occurs through optimal glycemic control, normotension, and avoidance of excess sodium and protein intake. Early detection and prompt intervention once complications occur is essential.
• Patient counseling, education, and motivation. Patient training programs in the following areas are vital for short- and long-term goal achievement: pathophysiology of DM and the prevention of complications; therapeutic options for optimal control and lifestyle flexibility; dietary instruction/counseling; exercise integration; foot care; and sick-day and minor-illness management. Patients must be well versed in integration of these principles into their daily lives.
MANAGEMENT OF TYPE 1 DIABETES MELLITUS: INSULIN CONSIDERATIONS
• Indications for outpatient initiation of insulin include the following: patient is not vomiting, has no evidence of clinical dehydration, has no evidence of diabetic ketoacidosis (DKA), and the necessary support staff are readily available. It is impossible to accurately predict insulin sensitivity based on weight alone. Conservative initial starting doses in an otherwise well patient are in the range of 0.25 to 0.5 units per kg of body weight.
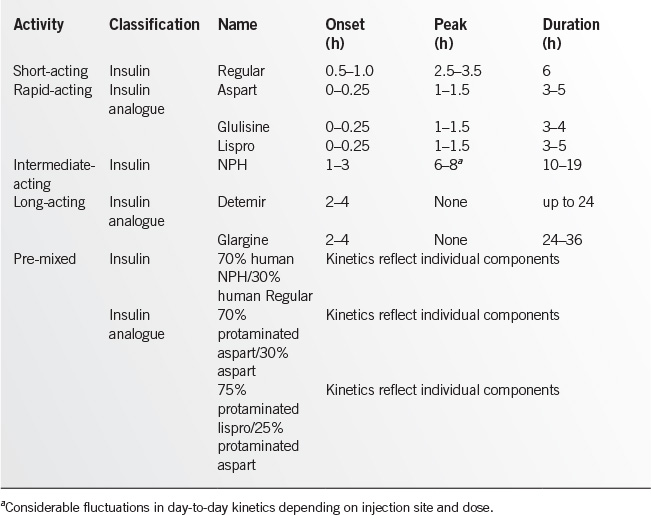
• Choice of insulin. Human insulin analogues and biosynthetic human insulin are the only insulins available in the United States. See Table 17.2-1 for insulin types and kinetics. The table reflects the kinetics seen in actual clinical practice rather than those reported by the manufacturers in nondiabetic individuals. Human insulin analogues are the preferred insulins except in cases in which cost is a major issue. Human biosynthetic insulins may be the only affordable option in this situation.
• Injection site principles are important for insulin absorption rate.
• Site selection
![]() Buttocks are the preferred site for bedtime injections in individuals still using human Neutral Protamine Hagedorn (NPH) insulin to minimize the risk of nocturnal hypoglycemia via (a) slower absorption, (b) avoidance of the 2 A.M. counterregulatory hormonal nadir, and (c) optimization of control of dawn surge in hepatic glucose output (dawn phenomenon).
Buttocks are the preferred site for bedtime injections in individuals still using human Neutral Protamine Hagedorn (NPH) insulin to minimize the risk of nocturnal hypoglycemia via (a) slower absorption, (b) avoidance of the 2 A.M. counterregulatory hormonal nadir, and (c) optimization of control of dawn surge in hepatic glucose output (dawn phenomenon).
![]() Upper abdomen is preferred for injections of rapid-acting insulin analogues (RAAs) due to (a) its more rapid insulin absorption and (b) better control of the early postprandial glucose level. Upper arms can be used as an alternative.
Upper abdomen is preferred for injections of rapid-acting insulin analogues (RAAs) due to (a) its more rapid insulin absorption and (b) better control of the early postprandial glucose level. Upper arms can be used as an alternative.
![]() Avoid RAA injections into legs and buttocks before meals due to slower absorption from these sites. On the other hand, exercise can accelerate the absorption of RAA from the legs and increase risk of hypoglycemia.
Avoid RAA injections into legs and buttocks before meals due to slower absorption from these sites. On the other hand, exercise can accelerate the absorption of RAA from the legs and increase risk of hypoglycemia.
• Site consistency. Patients must be instructed regarding the consistent use of anatomical regions for premeal and bedtime injections with adequate site rotation within these regions to prevent lipohypertrophy from repeated insulin injections in the same anatomic site. Injecting insulin into areas of lipohypertrophy can result in erratic insulin absorption and labile glucose levels. Injection site selection is less of an issue with the long-acting analogues glargine and detemir.
• Insulin injection timing issues
• Use of the subcutaneous site versus physiologic portal insulin results in delay in insulin absorption and an unavoidable mismatch between onset and peak of the insulin action and the onset and peak of blood glucose rise after carbohydrate ingestion.
• Injection interval
![]() Inject RAA insulins (aspart, glulisine, lispro) up to 10 minutes premeal, due to their rapid onset of action. Glulisine insulin injected up to 20 minutes after the start of a meal has been reported in a clinical trial to have similar kinetics to regular insulin injected 30 minutes premeal. This may be of benefit to individuals with unpredictable carbohydrate intake such as children, the elderly, and nursing home patients.
Inject RAA insulins (aspart, glulisine, lispro) up to 10 minutes premeal, due to their rapid onset of action. Glulisine insulin injected up to 20 minutes after the start of a meal has been reported in a clinical trial to have similar kinetics to regular insulin injected 30 minutes premeal. This may be of benefit to individuals with unpredictable carbohydrate intake such as children, the elderly, and nursing home patients.
![]() For individuals still using human regular insulin, injecting 30 to 40 minutes premeal helps to optimize postprandial glycemic control.
For individuals still using human regular insulin, injecting 30 to 40 minutes premeal helps to optimize postprandial glycemic control.
• A small snack (15 g carbohydrate) at the peak of the insulin action—3 hours for human regular insulin and 6 to 8 hours for human NPH—prevents hypoglycemia at this high-risk time.
• Intensive insulin therapy programs
• Multiple daily insulin injections
![]() Principles
Principles
– The use of multiple injections results in (a) smaller individual insulin doses, (b) more physiologic matching of carbohydrate and insulin, and (c) reduced risk of hypoglycemia. The better postprandial control that can be achieved with intensive insulin therapy (compared with conventional therapy) may reduce microvascular and macrovascular risk even with comparable HbA1c levels.
![]() Program options
Program options
– The qid+ program: (a) premeal/pre-snack dose of RAA insulin titrated to carbohydrate intake and (b) bedtime insulin glargine/detemir (40% to 50% total daily dose)
– Continuous subcutaneous insulin infusion (CSII) or insulin pump therapy
• Indications
• Failure of multiple daily insulin injection regimens
• Exuberant dawn phenomenon
• Need for convenience and flexibility
• Pregnancy
• Preconception
• Insulin pump setup
• RAA insulin is programmed at set hourly basal rates to control hepatic glucose output for the entire 24-hour day (approximately 40% to 50% total daily dose).
• Remainder of dose is premeal/snack RAA insulin titrated to carbohydrate intake.
• RAA insulin used nearly exclusively due to superior kinetics.
• Modern insulin pumps have algorithms that are programmed with insulin to carbohydrate ratios for carbohydrate insulin dose calculations. Additionally, there are algorithms for correction of hyperglycemia that involve target blood glucose levels, insulin sensitivity factors (the reduction in blood glucose level achieved for every additional unit of RAA administered), and insulin action time (the duration of a bolus of RAA). The patient enters into the pump his/her blood glucose level and the amount of carbohydrate to be consumed, and the pump calculates the appropriate amount of insulin to be delivered based upon the aforementioned parameters. The patient is prompted to accept/reject the calculated dosage. The hyperglycemia correction factors take into account residual insulin action from a prior bolus and reduce the risk of hypoglycemia resulting from overcorrection of hyperglycemia.
– The tid program with human NPH
• Two thirds of total daily dose is given in the morning. One-third of dose is RAA/human regular insulin. Two thirds of dose is human NPH insulin.
• One-third of total daily dose is given in the evening with half of the dose as RAA/human regular insulin before supper and half of the dose as human NPH given between 10 P.M. and 1 A.M.
• Ratios must be modified pending patient’s preferred mealtime carbohydrate distribution.
– A qid program can be used: (a) premeal dose (tid) of human regular insulin titrated to carbohydrate intake and (b) bedtime human NPH (20% of total daily dose).
– CAVEAT: There is NO place for pre-mixed insulin in the management of type 1 DM or pregnancy due to fixed ratios of rapid/intermediate-acting insulin and lack of flexibility.
• Insulin adjustments
• Baseline dose
![]() Doses of RAA, human regular, human NPH, glargine, or detemir insulin can be readily adjusted based on premeal, postprandial, and bedtime home blood glucose test results. Assuming a total daily dose of 0.5 unit per kg body weight, supplementing the baseline dose by 1 unit of insulin will drop an elevated blood glucose by approximately 50 mg per dL.
Doses of RAA, human regular, human NPH, glargine, or detemir insulin can be readily adjusted based on premeal, postprandial, and bedtime home blood glucose test results. Assuming a total daily dose of 0.5 unit per kg body weight, supplementing the baseline dose by 1 unit of insulin will drop an elevated blood glucose by approximately 50 mg per dL.
![]() Algorithm
Algorithm
– For the patient using supplemental RAA/human regular insulin:
• Use before meals and at bedtime. Example: In a patient taking 0.5 unit of insulin per kg body weight, the following calculations apply:
• Premeal and bedtime dosing. Add 1 unit of RAA/human regular insulin for every 50 mg per dL elevation in blood glucose above 100 mg per dL; that is, at 150 mg per dL, add 1 unit RAA/regular; at 200 mg per dL, add 2 units RAA/regular, etc.
• This should be revised pending individual patient glycemic goals and hypoglycemia risk.
• In the context of frequent follow-up, optimal control can be achieved with gradual insulin titration. Once achieved in the newly diagnosed patient, insulin requirements may gradually decline (“honeymoon phase”).
MANAGEMENT OF TYPE 1 DIABETES MELLITUS: DIETARY CONSIDERATIONS
• Basic principles. Current emphasis is carbohydrate counting while decreasing saturated fats and trans-fats, increasing dietary fiber and moderating protein intake.11 Most commonly, carbohydrates account for 45% to 60% of total daily calories, fat should account for less than 30%, and protein for 15% to 35% of total daily calories. Better matching of insulin to intake of total carbohydrate through gram counting of carbohydrate allows the incorporation of modest amounts of sucrose in the diet. The use of a carbohydrate counting book/smartphone apps and food scales is essential for accurate carbohydrate counting and subsequent insulin dosing.
• Priorities for patient education
• Emphasize carbohydrate counting and consistency with three meals. If patients are using human regular or human NPH insulin, add 15 g carbohydrate snacks to cover insulin peaks, in the context of an appropriate total caloric intake.
MANAGEMENT OF TYPE 1 DIABETES MELLITUS
Home blood glucose monitoring (HBGM) considerations:
• Basic principles
• Accuracy in HBGM is critical to safety and success of intensive therapy.
• Frequency
![]() Minimum of four tests per day should be done before meals and at bedtime.
Minimum of four tests per day should be done before meals and at bedtime.
![]() Monitoring 2 hours after a meal is necessary to accurately determine the adequacy of the premeal RAA insulin doses and the timing of the dose. Individuals who slowly absorb insulin may need to administer their RAA doses as far as 20 to 30 minutes premeal/snack to optimize postprandial glycemic control.
Monitoring 2 hours after a meal is necessary to accurately determine the adequacy of the premeal RAA insulin doses and the timing of the dose. Individuals who slowly absorb insulin may need to administer their RAA doses as far as 20 to 30 minutes premeal/snack to optimize postprandial glycemic control.
![]() Additional testing is done when hypoglycemia or hyperglycemia is suspected.
Additional testing is done when hypoglycemia or hyperglycemia is suspected.
![]() Periodic 2 A.M. and 4 A.M. tests
Periodic 2 A.M. and 4 A.M. tests
![]() Prior to driving if symptomatic
Prior to driving if symptomatic
• Patients must follow manufacturer’s instructions carefully to achieve accurate results.
• Sources of error in HBGM: Improper cleansing of finger; failure to wipe away first drop of blood when alcohol is used to cleanse finger; volume of blood applied to strip is too much or too little; meter is not calibrated to strip lot number; damaged strips resulting from exposure to light, heat, cold or humidity; out-of-date strips; meter not properly cleaned; and failure to use glucose control solutions to verify strip accuracy. Patient precision is vital to successful use of the insulin algorithm and sick-day management.
• Glucose sensors which measure interstitial glucose levels are an adjunct to, not a replacement for, HBGM. The sensors provide trend detection in regard to the direction of change in glucose levels via a visual display and should not be used as a substitute for HBGM. Additionally, audible alarms signal when the glucose level has reached a preprogrammed rate of fall or rise as well as when a prespecified glucose level is reached. Appropriate candidates are individuals with type 1 DM on physiologic insulin programs, are attentive to counting grams of carbohydrate, and who attend regularly for their follow-up appointments. In addition to a visual display, audible alarms signal when the interstitial glucose level reaches a prespecified rate of fall or rise in glucose as well as when a prespecified interstitial glucose level is attained. One insulin pump system, which is integrated with a glucose sensor, can be programmed to suspend basal insulin delivery for up to 2 hours in event of a hypoglycemia threshold being reached.
MANAGEMENT OF TYPE 1 DIABETES MELLITUS: EXERCISE CONSIDERATIONS
• Physical fitness is a goal for all individuals with DM. Safe exercise plans must be individualized based on age, cardiovascular status, foot problems, neuropathy, and retinopathy. Even increased activity, such as grocery shopping, results in a lowering of blood glucose levels. Uncompensated physical activity is a very common cause of hypoglycemia in the patient with type 1 DM.
• Insulin adjustment for exercise or activity. Use for planned physical activity.
• Reduce the RAA insulin dose that is active during the exercise by 1 to 2 units or more for every 20 to 30 minutes of exercise. The amount of reduction will depend upon the intensity of the exercise.
• Occasionally, individuals have a delayed or sustained response to physical activity such that their bedtime insulin dose may need to be reduced by 1 to 2 units or more following, for example, evening physical activity. Again, the amount of reduction will depend upon the intensity of the exercise.
• Insulin pump patients have the option to program a temporary reduction in their basal insulin infusion rates. Temporary basal rates must be programmed 45 to 60 minutes prior to the planned physical activity due to the kinetics of subcutaneous insulin.
• Carbohydrate adjustment for exercise or activity
• Use for either planned or spontaneous activity.
• Augment carbohydrate intake as follows: Add a 15-g carbohydrate snack for every 20 to 30 minutes of physical activity, depending on the intensity of activity.
STANDARD OF CARE FOR FOLLOW-UP
Once glycemic control has been established, maintenance of glycemic control depends on the frequency of follow-up.
• Minimum visit frequency is once every 3 months.
• Review history; perform an interim physical examination; identify patient errors and omissions; adjust the patient’s insulin dosage, diet, and exercise program; and do ongoing patient counseling. Laboratory evaluations should include HbA1c every 3 months and annual assessments of urine microalbumin/creatinine ratio, thyroid-stimulating hormone, blood chemistries, and a lipid panel. In addition, an electrocardiogram (EKG) and ankle–brachial indices (see following section) may be obtained annually or sooner depending on the patient symptomatology.
DAWN PHENOMENON
• The dawn phenomenon is a markedly elevated fasting blood glucose secondary to an exuberant rise in hepatic glucose output. This hyperglycemia results from a surge in counterregulatory hormone concentrations (growth hormone, catecholamines, and cortisol) in the absence of nocturnal hypoglycemia.
• Treatment
• Delaying the timing of bedtime insulin dose until closer to midnight and titrating up the bedtime NPH, glargine, or detemir insulin usually suffices. In some instances, however, the dose increase results in hypoglycemia prior to the dawn surge. CSII is ideal in this situation, such that basal rates can be preprogrammed to coincide with the patient’s individual dawn surge. Glucose sensor trends can be helpful in this regard.
AFTERNOON PHENOMENON
• The afternoon phenomenon is late afternoon/pre-dinner hyperglycemia in the setting of good glycemic control postlunch. In patients with insulin pumps, afternoon basal rates can be increased to accommodate this. In patients on MDI, adding a pre-breakfast or sometimes even a prelunch dose of basal insulin can help to control this.
SOMOGYI PHENOMENON
• The Somogyi phenomenon is post-hypoglycemia hyperglycemia due to a surge in counterregulatory hormones, rather than insulin “run-out” or overtreatment of hypoglycemia with excess carbohydrate.
• Strategy. Perform HBGM at 2 A.M. and 4 A.M. in addition to premeal and bedtime HBGM. If the Somogyi phenomenon is identified, a dose reduction of the evening basal insulin is indicated, coupled perhaps with a shift in the timing of the injection as late as possible (midnight or thereafter), with emphasis on the lower buttocks as the injection site of choice if human NPH insulin is being used. Glucose sensor trends can be helpful in this regard.
HYPOGLYCEMIA
Recurrent. In a well-designed, physiologic, individualized treatment program, most episodes of hypoglycemia are related to patient error.
• Patient-related errors include insulin–carbohydrate mismatch, delayed or missed meals, missed snacks, uncompensated exercise, erratic insulin injection site rotation, injecting into areas of lipohypertrophy, and lack of adequate HBGM (inaccurate tests or low frequency of HBGM).
• Nonpatient-related problems include unpredictable absorption or kinetics of basal insulin or, less frequently, insulin autoantibodies. This is best treated by utilizing a true basal-bolus insulin regimen with insulin glargine or detemir along with RAA.
• Hypoglycemia unawareness
• Hypoglycemia can be a major problem in intensive therapy and a limitation to achieving glycemic targets. In most cases, it is reversible to varying degrees through program revisions designed to eliminate hypoglycemia.
• Once hypoglycemia has been eliminated for a period of 3 to 6 weeks, the patient’s subjective awareness and counterregulatory hormonal response will improve, with the exception of the glucagon response. Improvement in hypoglycemia awareness facilitates safe lowering of ambient glucose levels and HbA1c.
• These patients may be very good candidates for glucose sensors assuming patient adherence to all aspects of an intensive insulin therapy regimen and pending health insurance coverage.
DIABETIC KETOACIDOSIS IN ADULTS
Prevention and management. DKA is a syndrome of hyperglycemia, ketonemia, and ketonuria of varying intensity that results in death in 10% of cases.
• Causes. Minor illnesses are the most common cause, such as upper respiratory and urinary tract infections. Major illnesses (such as myocardial infarction [MI] and major sepsis infarction) are a less common cause. Most cases of severe DKA can be averted through aggressive attention to the sick-day management guidelines (refer to Special Issues, below).
• Treatment
• If intractable emesis occurs, take the following measures:
![]() Administer early intravenous hydration with 1 to 2 L of normal saline fluids in emergency room.
Administer early intravenous hydration with 1 to 2 L of normal saline fluids in emergency room.
![]() Administer SC insulin bolus, not IV insulin bolus, as the half-life of an IV bolus of regular insulin is only 5 minutes.
Administer SC insulin bolus, not IV insulin bolus, as the half-life of an IV bolus of regular insulin is only 5 minutes.
![]() Administer parenteral or rectal antiemetics.
Administer parenteral or rectal antiemetics.
![]() Do not delay. Delay in seeking therapy is the major factor in severe DKA episodes, which result in costly hospitalizations in intensive care units and even death.
Do not delay. Delay in seeking therapy is the major factor in severe DKA episodes, which result in costly hospitalizations in intensive care units and even death.
• Identify and address the underlying illness. Rule out silent MI or occult sepsis as cause of DKA.
• Correct the volume depletion.
![]() Give 1 to 2 L of normal saline in the first 1 to 2 hours to correct hypotension and establish good urine output. In children and adolescents, give 500 mL of normal saline per hour for the first 1 to 2 hours.
Give 1 to 2 L of normal saline in the first 1 to 2 hours to correct hypotension and establish good urine output. In children and adolescents, give 500 mL of normal saline per hour for the first 1 to 2 hours.
![]() Total volume deficit is frequently 5 to 6 L. In most individuals, volume can be replaced over 12 to 24 hours, depending on the underlying cardiac and renal status.
Total volume deficit is frequently 5 to 6 L. In most individuals, volume can be replaced over 12 to 24 hours, depending on the underlying cardiac and renal status.
![]() Failure to adequately rehydrate and correct ketosis completely is a common cause of rapid DKA relapse despite correction of hyperglycemia. Patients need to be ketone free for 24 hours to ensure complete correction.
Failure to adequately rehydrate and correct ketosis completely is a common cause of rapid DKA relapse despite correction of hyperglycemia. Patients need to be ketone free for 24 hours to ensure complete correction.
• Insulin treatment. Start a low-dose IV infusion at the rate of 0.1 unit/kg/hour to result in a 100 mg/dL/hour fall in blood glucose. Adequately dilute the insulin to allow fine titration (50 units human regular insulin in 500 mL normal saline). This dosage overcomes the common clinical problem of having 1 unit per hour be the lowest infusion rate possible. This is especially important for very insulin–sensitive patients. There is no place for RAA in IV insulin therapy. To maintain a sufficiently high insulin dose to correct ketosis without hypoglycemia, the intravenous fluids must be changed to dextrose 5% or 10% when blood glucose level falls below 250 mg per dL.
• Electrolyte replacement
![]() Potassium replacement may be initiated once the serum potassium is <5.5 mEq per L and urine output is documented. Use 20 to 40 mEq per L IV fluids and monitor serum potassium values q2h.
Potassium replacement may be initiated once the serum potassium is <5.5 mEq per L and urine output is documented. Use 20 to 40 mEq per L IV fluids and monitor serum potassium values q2h.
![]() Bicarbonate therapy is controversial and is rarely used unless there is severe acidosis with hemodynamic instability or severe hyperkalemia with EKG changes. Clinical trials have failed to demonstrate any benefit in the treatment of DKA. Bicarbonate should not be given by IV push because that could result in cerebral edema, especially in children, which is often fatal.
Bicarbonate therapy is controversial and is rarely used unless there is severe acidosis with hemodynamic instability or severe hyperkalemia with EKG changes. Clinical trials have failed to demonstrate any benefit in the treatment of DKA. Bicarbonate should not be given by IV push because that could result in cerebral edema, especially in children, which is often fatal.
![]() Phosphate repletion has more theoretical than proven practical benefits unless severe depletion is present (serum phosphorus <0.5 mg per dL).
Phosphate repletion has more theoretical than proven practical benefits unless severe depletion is present (serum phosphorus <0.5 mg per dL).
![]() Magnesium repletion. If deficiency is severe (serum magnesium level <1.0 mEq per L) or the patient is symptomatic (seizures, tetany, cardiac arrhythmias), then replace with magnesium chloride at a dose of 1 mEq/kg/24 hour, assuming normal renal function.
Magnesium repletion. If deficiency is severe (serum magnesium level <1.0 mEq per L) or the patient is symptomatic (seizures, tetany, cardiac arrhythmias), then replace with magnesium chloride at a dose of 1 mEq/kg/24 hour, assuming normal renal function.
• Monitor DKA progress. Clinical and laboratory monitoring of the patient should be documented on a flow sheet. Laboratory parameters should be followed at least every 2 hours until stability emerges.
HYPERGLYCEMIC HYPEROSMOLAR NONKETOTIC COMA
• Characteristics. This condition occurs in type 2 DM patients, most commonly with underlying renal insufficiency or cerebrovascular disease (cerebrovascular accident or subdural hematoma). The degree of dehydration is more severe than that of DKA. Blood glucose levels range from 600 to 2,000 mg to dL, and ketosis is generally absent.
• Treatment is similar to that for DKA in terms of IV fluids, insulin, and electrolytes, but hydration rates must be lower.
• The initial infusion rate of normal saline should not exceed 1 L per hour to expand the extracellular space, with the IV fluid being switched to half normal saline once blood pressure is stable and good urine output is established. Fluid should be replaced over a 24-hour period.
• Often insulin therapy is not needed on an ongoing basis once the acute metabolic derangement has been corrected and any underlying precipitating illness treated or resolved.
INITIAL MANAGEMENT OF TYPE 2 DIABETES MELLITUS
• Minimally decompensated presentation
• Clinical picture includes obesity and mild to moderate hyperglycemia with or without symptoms. Treatment strategies include patient education, training, and motivation; an individualized hypocaloric meal plan (regardless of the macronutrient distribution)12 with dietary counseling; and an exercise plan tailored to the individual. Emphasize permanent lifestyle modification. Additionally, metformin is advocated as a component of initial therapy, assuming no contraindications.
• Moderately decompensated presentation
• Clinical picture. Obesity, severe symptomatic hyperglycemia (fasting blood glucose >300 mg per dL), and mild dehydration or decompensation call for more urgent lowering of blood glucose levels, largely for symptomatic relief and reversal of the glucotoxic effect of the prior sustained hyperglycemia on pancreatic islet insulin secretion and peripheral insulin action.
• Treatment strategies
• Temporary insulin therapy with daily glargine/detemir at a starting daily dose of 0.4 units per kg of weight and an algorithm for hyperglycemia similar to that for type 1 DM patients will rapidly yield symptomatic relief and reversal of islet and peripheral/target organ glucotoxicity.
• Severely decompensated presentation
• Clinical picture shows a severely symptomatic patient with blood glucose levels frequently exceeding 350 mg per dL, marked dyslipidemia (serum triglycerides >1,000 mg per dL), and hyperosmolality with absence of ketosis.
• Treatment strategies
• Intravenous fluids and insulin (similar to DKA) in the hospital setting are necessary for acute reversal of the metabolic derangement, followed later by a switch to daily insulin glargine/detemir.
• Start patient training and education with an individualized meal plan,11 dietary counseling and an exercise plan. The long-term goal may be tapering and withdrawal of insulin, pending residual insulin secretion, assuming that glycemic control can be maintained with diet, exercise, oral agent therapy and/or injectable incretin-based therapy.
• Special situation: The nonobese type 2 diabetic patient. Patients who are at less than 120% of IBW comprise approximately 10% of those with type 2 DM. It is important to rule out LADA in these individuals. If LADA is ruled out, these patients may benefit from modest weight loss toward IBW, with a hypocaloric meal plan (regardless of the macronutrient distribution),12 dietary counseling, and exercise training program. Exercise is especially beneficial to these patients, many of whom are relatively insulinopenic and poor responders to oral agents. Frequently these patients will require basal insulin therapy with glargine/detemir insulin, and many will need to progress to full insulin replacement akin to an individual with type 1 DM.
DIETARY MANAGEMENT OF TYPE 2 DIABETES MELLITUS
• Goals in overweight and obese patients
• Reduction in body weight
![]() Strategies. Reduce overall caloric intake (regardless of the macronutrient distribution)12 through carbohydrate counting and fat gram counting. Consumption of 100 kcal per day over and above caloric needs will result in a 10-pound annual weight gain. Reduction in daily caloric intake by 500 calories daily will facilitate a 1-pound weekly weight loss.
Strategies. Reduce overall caloric intake (regardless of the macronutrient distribution)12 through carbohydrate counting and fat gram counting. Consumption of 100 kcal per day over and above caloric needs will result in a 10-pound annual weight gain. Reduction in daily caloric intake by 500 calories daily will facilitate a 1-pound weekly weight loss.
![]() A reduction of even 5% to 10% in weight can have a major impact on the clinical course of a type 2 DM patient. Patients need not reduce to IBW to achieve euglycemia, but the closer they are, the better all the other markers of the metabolic syndrome will be (e.g., dyslipidemia, hypertension).
A reduction of even 5% to 10% in weight can have a major impact on the clinical course of a type 2 DM patient. Patients need not reduce to IBW to achieve euglycemia, but the closer they are, the better all the other markers of the metabolic syndrome will be (e.g., dyslipidemia, hypertension).
![]() Weight loss results in removal of fat from the liver, pancreas, and skeletal muscle and improving insulin sensitivity.
Weight loss results in removal of fat from the liver, pancreas, and skeletal muscle and improving insulin sensitivity.
EXERCISE THERAPY FOR TYPE 2 DIABETES MELLITUS
All of the benefits of exercise for the type 1 DM patient apply even more to the type 2 DM patient, with the added benefit of raising the frequently depressed high-density lipoprotein cholesterol (HDL-C) level. Adherence to an ongoing exercise routine is one of the most powerful predictors of maintenance of weight loss. The importance of exercise capacity and survival must be stressed. In the LOOK-AHEAD trial, intensive lifestyle modification resulted in a reduction in HbA1c of 0.7% after 1 year, equivalent to many pharmacotherapeutic agents.
PHARMACOTHERAPY OF TYPE 2 DIABETES MELLITUS
• Introduction
• Pharmacotherapy of type 2 DM is now directed at the specific pathophysiologic defects that have been identified as the ominous octet:13
![]() Increased hepatic glucose production
Increased hepatic glucose production
![]() Decreased insulin secretion
Decreased insulin secretion
![]() Decreased skeletal muscle glucose uptake
Decreased skeletal muscle glucose uptake
![]() Hypersecretion of glucagon
Hypersecretion of glucagon
![]() Diminished incretin effect
Diminished incretin effect
![]() Increased lipolysis
Increased lipolysis
![]() Increased renal glucose reabsorption
Increased renal glucose reabsorption
![]() Neurotransmitter dysfunction in the central nervous system
Neurotransmitter dysfunction in the central nervous system
![]() All oral medications can be used as initial therapy, or as second- or third-order therapy in combination with other oral agents, injectable incretin-based therapy or insulin.
All oral medications can be used as initial therapy, or as second- or third-order therapy in combination with other oral agents, injectable incretin-based therapy or insulin.
• Insulin sensitizers
• Metformin
![]() Indication. Metformin therapy is indicated as first-line therapy for all patients with type 2 DM who are free from significant liver disease, have a serum creatinine <1.4 mg per dL in women or <1.5 mg per dL in men. Lactic acidosis is a risk if any of these underlying conditions exist. Metformin should be discontinued on the day of any iodinated dye-load procedure or surgery and should be withheld for 48 hours after the procedure. Metformin can be restarted until a normal serum creatinine is confirmed post-contrast study/surgery. Metformin should also be withheld during treatment for pneumonia or acute MI.
Indication. Metformin therapy is indicated as first-line therapy for all patients with type 2 DM who are free from significant liver disease, have a serum creatinine <1.4 mg per dL in women or <1.5 mg per dL in men. Lactic acidosis is a risk if any of these underlying conditions exist. Metformin should be discontinued on the day of any iodinated dye-load procedure or surgery and should be withheld for 48 hours after the procedure. Metformin can be restarted until a normal serum creatinine is confirmed post-contrast study/surgery. Metformin should also be withheld during treatment for pneumonia or acute MI.
![]() Mode of action. Metformin is an insulin sensitizer at the level of the liver with a primary mode of action of controlling excess hepatic glucose output. It is not a hypoglycemic agent; therefore, it generally cannot cause hypoglycemia when used as monotherapy.
Mode of action. Metformin is an insulin sensitizer at the level of the liver with a primary mode of action of controlling excess hepatic glucose output. It is not a hypoglycemic agent; therefore, it generally cannot cause hypoglycemia when used as monotherapy.
![]() Dosing
Dosing
– Use as monotherapy or in combination with other oral agents, injectable incretin-based therapy, and/or insulin.
– Initial dose
• Immediate release (IR) (500 mg bid with food) can be titrated to a maximum of 2,550 mg in an 850-mg tid dosing schedule. Maximum effective dose is generally seen at 1,000 mg bid.
– Extended-release (ER) preparations can be used to facilitate dosing convenience and reduce the risk of diarrhea. The initial dose is 500 mg with the evening meal titrated to 2,000 mg daily.
![]() Other effects. Metformin facilitates weight loss and can improve the lipid profile.
Other effects. Metformin facilitates weight loss and can improve the lipid profile.
• Adverse effects. Gastrointestinal (GI) side effects of nausea, flatus, and diarrhea can occur but are usually self-limited (1 to 2 weeks). Side effects can be minimized by taking medication with food and using an ER preparation. Long-term discontinuation rate due to GI side effects is generally less than 4%. Vitamin B12 malabsorption may occur.14
• Thiazolidinediones (glitazones)
![]() Indications.
Indications.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree