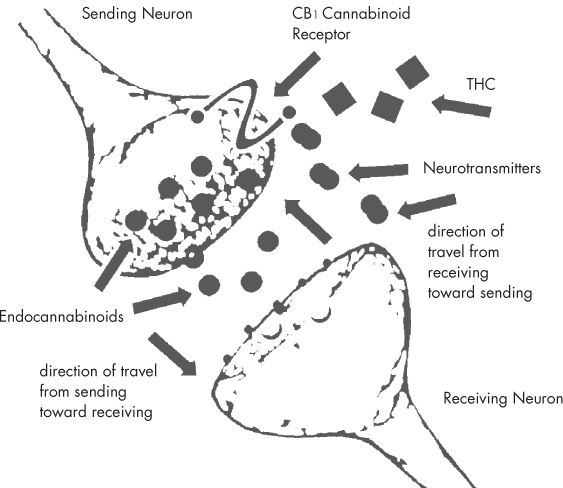
Humans have used cannabis for centuries, but only in the last 50 years or so has any scientific understanding emerged as to how cannabis works within the human body. While the discovery of the first plant cannabinoids took place in the 1940s, it was not until 1964 that THC produced by the cannabis plant was first characterized and synthesized. The discovery of THC in 1964 (see this page) led to a long search for a receptor in the body with which THC might be interacting. The first cannabinoid receptors in the body were not characterized until the late 1980s. These receptors turned out to comprise a new series of regulatory mechanisms within the body, called the endocannabinoid system. Many of the physiological effects of cannabis occur due to the interaction of cannabinoids with the endocannabinoid receptor molecules. The endocannabinoid system consists of this network of endocannabinoid receptors, which are distributed throughout the body. The system is a very complex regulatory system, broad in its function, and found within all complex animals, from fish to humans. The endocannabinoid system supports such diverse functions as memory, digestion, motor function, immune response, appetite, pain, blood pressure, bone growth, and the protection of neural tissues. Many researchers believe that there are even more physiological processes with which the endocannabinoid system is involved, still yet to be discovered. The two primary subtypes of cannabinoid receptor in the endocannabinoid system are CB1 and CB2. These receptors are distributed throughout the central nervous and immune systems, and within many other tissues, including the brain, gastrointestinal system, reproductive and urinary tracts, spleen, endocrine system, heart, and circulatory system. Furthermore, researchers have uncovered new evidence that points to at least three other cannabinoid receptors throughout the body, in addition to CB1 and CB2. Following the discovery of the cannabinoid receptors, the hunt was launched for the substances produced within the body that were binding to them. This led to the discovery of the first endocannabinoids, anandamide and 2-AG, in the early 1990s. So far, five endocannabinoids have been isolated. All of them are derivatives of polyunsaturated fatty acids, closely related to the popular omega-3 fatty acids (which are often purchased as supplements from health stores). Since they are fats, endocannabinoids are not water-soluble and have difficulty moving efficiently through the body; thus, they are designed to work locally. One local activity occurs when endocannabinoids serve as the primary messenger across synapses (the gaps between nerve cells): they signal neurons to communicate with each other through the release of neurotransmitters. In recent years, it has become increasingly clear that the role of endocannabinoids in this synaptic function is both more important and far more complex than previously thought. Endocannabinoids modulate the flow of neurotransmitters, keeping our nervous system running smoothly. Endocannabinoids are produced on demand, released back across the synapse, then taken up into the cells and rapidly metabolized. Endocannabinoids appear to be profoundly connected with the concept of homeostasis (maintaining physiological stability), helping redress specific imbalances presented by disease or by injury. Endocannabinoids’ role in pain signaling has led to the hypothesis that endocannabinoid levels may be responsible for the baseline of pain throughout the body, which is why cannabinoid-based medicines may be useful in treating conditions such as fibromyalgia (a condition marked by muscular pain and stiffness). This could also mean that the constant release of the body’s own endocannabinoids could have a “tonic” effect on muscle tightness (spasticity) in multiple sclerosis, neuropathic pain, inflammation, and even baseline appetite. The value of proper “endocannabinoid tone” throughout the body could be very significant to general well-being. While CB1 activation results in the psychological and physical effects commonly associated with cannabis ingestion, CB2 receptor activation does not. The CB2 receptors are primarily found in blood cells, tonsils, and the spleen. From these sites, CB2 receptors control the release of cytokines (immunoregulatory proteins) linked to inflammation and general immune function throughout the body. The endocannabinoid system as a target for drug delivery goes well beyond the use of cannabis. Cannabinoid-based medicines can either enhance or interfere with the endocannabinoid system’s balancing act. But designing drugs that interact safely with the endocannabinoid system is difficult, and drugs that antagonize or interfere with the function of cannabinoid receptors have met with decidedly mixed success. Rimonabant, a CB1 antagonist, was approved for sale in Europe in 2006 as an obesity treatment. However, the Food and Drug Administration refused to approve it in the U.S., citing concerns that the drug was linked to episodes of depression and suicidal behaviors. Because the cannabinoid receptors are dispersed so widely throughout the body, activating or suppressing them for a single medical purpose can unleash a host of unwanted activity elsewhere. Recent research has started to focus on developing drugs that can interact with cannabinoid receptors, but that do not cross the blood/brain barrier, in hopes of preventing some serious side effects. As mentioned above, Rimonabant, which blocked the effects of the CB1 receptor in hopes of reducing appetite as a diet drug, was removed from the market because of psychiatric side effects. However, it is believed that these types of side effects might be reduced by limiting the ability of these new drugs to block or interact with these receptors. Other drug candidates seek to slow down how quickly anandamide—one of the key endocannabinoids—is metabolized. These proposed drugs show promise as treatments for conditions ranging from cancer to colitis.
THE ENDOCANNABINOID SYSTEM: A BRIEF PRIMER
Cannabinoid Receptors
Basicmedical Key
Fastest Basicmedical Insight Engine