Learning Objectives
- Recognize emerging and reemerging infectious diseases as threats to global health
- Raise awareness of the national and international response
- Learn about global issues of antimicrobial resistance of a variety of organisms and their spread
- Understand various concepts of antimicrobial resistance
- Learn about the impact of antimicrobial resistance among patients and communities
- Expand knowledge regarding prevention and control strategies directed against problems of antimicrobial resistance
Case Study of an Emerging Infection
Severe acute respiratory syndrome (SARS) is a prototypical emerging infectious disease that, largely because of global travel, instead of remaining an obscure respiratory infection in South China became a global public health crisis. By the time the outbreak ran its course, over 8,000 cases were identified from 29 countries with an overall 10% fatality rate.
Global attention toward the outbreak was first drawn in March 2003 with the recognition of cases of severe acute respiratory illness among patients in the Guangdong province of China, Hong Kong, Vietnam, Singapore, and Canada. The World Health Organization (WHO) issued a global alert and coined the term severe acute respiratory syndrome (SARS) for the disease. By April 2003, the WHO had to take the unprecedented step of issuing a travel advisory for the Guangdong Province and for Hong Kong, later broadened to other countries. Eventually, the etiologic agent was identified as a novel coronavirus (SARS CoV), likely a virus that jumped species from the civet (catlike delicacy in China) to humans. The initial zoonotic transmission was followed by subsequent nosocomial and human-to-human transmission perpetuating a widespread global epidemic. Most patients presented with fever, cough, shortness of breath, and reported either close contact with a person with SARS or a history of travel or residence in an area with recent local SARS transmission. The chest radiograph would reveal findings of pneumonia or acute respiratory distress syndrome, with some cases progressing on to require ventilatory support. Supportive treatment remained the mainstay of clinical care.
Initial cases of SARS were reported from Guangdong Province, China, in November 2002 with almost 800 cases noted by February 2003. A physician with SARS contributed to the subsequent widespread dissemination of the disease by traveling from Guangdong to a hotel in Hong Kong and infecting 10 other individuals who then traveled widely, perpetuating outbreaks in their countries of destination.1 Most severe illness occurred in adults, with children, if infected at all, developing a milder illness. Patients older than 60 years had a higher mortality, with a case fatality rate up to 43%. Twenty-nine countries in Asia, Europe, and North America were affected with 83% of the reported cases hailing from China and Hong Kong. Table 12-1 depicts the timeline of the SARS outbreak.
Nov. 2002 Feb. 2003 March 2003 April 2003 May 2003 June 2003 July 2003 | First cases reported in Southern China Up to 792 cases reported from Guangdong Province, China Index case of symptomatic physician traveling to Hong Kong Transmission to others living in same hotel as index case Widespread dissemination to Singapore, Vietnam, Canada, Thailand Global alert issued by WHO (March 12) 1,622 cases reported from 13 countries, with 58 deaths WHO issues travel advisory to China, Hong Kong, Taiwan, and Toronto 5,663 cases reported from 26 countries, with 372 deaths Travel advisories lifted for Toronto 8,360 cases reported from 29 countries, with 764 deaths WHO lifts last remaining travel advisory for China 100 days into the outbreak (June 19) 8,447 cases reported from 29 countries, with 811 deaths Major epidemic in Asia ended WHO declares SARS outbreak contained worldwide |
Although most disease transmission occurred by droplet spread (requiring face-to-face contact), airborne transmission with droplet nuclei was strongly suspected as the cause of cases in a large apartment complex in Hong Kong.2 Transmission to health care workers was a common feature of the outbreak. This was likely precipitated by the high levels of viral shedding in nasopharyngeal aspirates early, and in stool later in the disease course, with possible environmental contamination. Infection control guidelines requiring that hospitalized patients be isolated in negative pressure rooms and that all health care workers wear masks, gowns, gloves, and protective eyewear helped finally control the epidemic.
Definition and Background of Emerging Diseases
The global spread of infectious disease can be traced back to the 16th century when Spanish explorers reportedly introduced smallpox, typhus, and measles to the susceptible population of the New World and returned with syphilis. This introduction of new diseases resulted in catastrophic depopulation with approximately 50 million deaths among the native South Americans. There have subsequently been a number of major epidemics, but by the mid-20th century, primarily with the rapid advances in sanitation and public health, infectious diseases were believed to be a problem of the past. In the United States, infectious disease mortality rates, per 100,000 population, fell from 500 in 1900 to 50 in 1960.3 This success was reflected in statements such as “one can think of the middle of the twentieth century as the end of one of the most important social revolutions in history, the virtual elimination of infectious disease as a significant factor in social life.”4
This optimism, unfortunately, was short lived as infectious diseases staged a dramatic comeback with more than 30 new diseases, including the HIV/AIDS pandemic, emerging in just the past 4 decades.5 In addition, old foes like malaria and tuberculosis threatened to return with a vengeance. Among these and other infections, our options for control started shrinking as drug resistance started spreading. In fact, in the United States, between 1980 and 1992, the death rate from infectious diseases increased 50%.3 Attention toward these emerging and reemerging infections was first drawn by a landmark 1992 report by the Institute of Medicine.6 This report drew attention to the fact that pathogenic microbes can be resilient and dangerous foes. Although it is impossible to predict their individual emergence in time and place, we can be confident that new microbial diseases will continue to emerge. Based on this report, these diseases were defined as “New, reemerging or drug-resistant infections whose incidence in humans has increased within the past two decades or whose incidence threatens to increase in the near future.”
This concept of emerging infections is flexible, reflecting not only the temporal and geographic interactions between humans and microbes, but also the ability of the medical community to identify them. The relationship between humans and microbe is seldom stable. New threats are ever present, confronting public health authorities as well as physicians.
The Institute of Medicine report identified some key factors explaining why infectious diseases emerge or reemerge. These include the following:
Global travel
Globalization of the food supplies and centralized processing of food
Population growth and increased urbanization and crowding
Population movements due to civil wars, famines, and other human-made or natural disasters
Irrigation, deforestation, and reforestation projects that alter the habitats of disease-carrying insects and animals
Human behaviors, such as intravenous drug use and risky sexual behavior
Increased use of antimicrobial agents and pesticides, hastening the development of resistance
Increased human contact with tropical rain forests and other wilderness habitats that are reservoirs for insects and animals that harbor unknown infectious agents
Year | Infectious agent identified |
|---|---|
1975–1979 | Parvovirus B19, Cryptosporidium parvum, Ebola, Legionella pneumophila, Campylobacter spp. |
1980–1984 | Borrelia burgdorferi, HIV, Escherichia coli 0157:H7, Helicobacter pylori |
1985–1989 | Ehrlichia spp., hepatitis C and E virus |
1990–1994 | Vibrio cholera 0139, Bartonella henselae, Sin nombre virus, human herpes virus 8/Kaposi sarcoma, herpes virus |
1995–1999 | Prions, influenza A H5N1, enterovirus 71, West Nile virus |
2000–2004 | SARS-coronavirus, human metapneumovirus |
2005–2009 | Pandemic influenza A H1N1 |
Although there are many examples of new, emerging, and reemerging illnesses, only those of particular importance worldwide are discussed here. Agents of bioterrorism have assumed great recent importance and are also included.
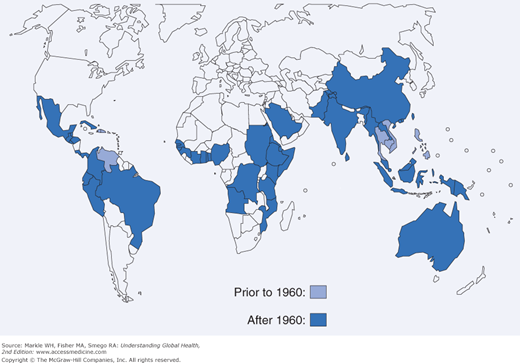
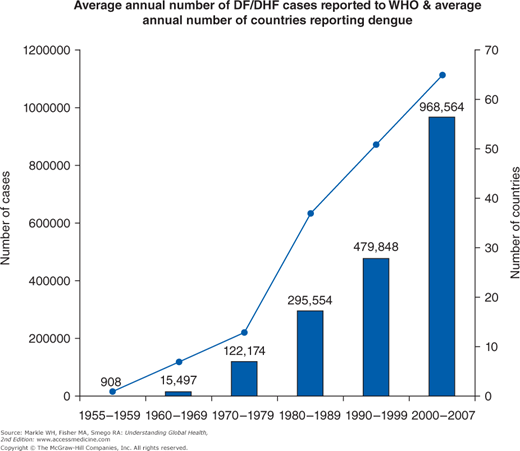
Dengue virus infection is an example of an emerging infection with a wide distribution, now found in all continents except Europe and Antarctica (Figures 12-1 and 12-2). With over 100 million annual infections worldwide among the 2.5 billion individuals at risk, the dengue viruses are arguably the most important arthropod-borne viruses from a medical and public health perspective.7 A flavivirus, there are four antigenically related but distinct dengue virus serotypes carried by the principal mosquito vector Aedes aegypti that is well adapted to the urban environment.
Both epidemic and endemic transmission is maintained through a human-mosquito-human cycle, and no evidence for a significant animal reservoir exists, unlike yellow fever or West Nile virus. Susceptible individuals become infected after a bite from the infected female Aedes mosquito and become viremic toward the end of a 4- to 6-day incubation period. Viremia persists until the resolution of fever, usually in 7 days. Mosquitoes become infected if they feed on the viremic individual, and after 8 to 12 days are capable of transmission of disease for the duration of their lifespan. They are daytime urban feeders and prefer to bite humans, frequently taking multiple blood meals in a single breeding cycle. Thus transmission among multiple family members is common. Aedes aegypti are widely distributed from latitude 45° N to 35° S. Although greatly restricted in distribution in the Western Hemisphere during the 1970s yellow fever control programs, they now have reinfested nearly all their former habitats.
The vast majority of cases of dengue virus infection globally are from areas with hyperendemic transmission: the continuous circulation of multiple dengue virus serotypes, particularly in urban areas. Most clinical cases occur among children because the prevalence of antibody rises with age, and most adults are immune. This immunity, however, is serotype specific and may, in fact, predispose to more fulminant disease. Instead of a self-limiting, nonspecific, febrile viral illness, individuals with antibodies to one dengue virus serotype but infected with another serotype are more likely to manifest the complications of hemorrhagic fever and dengue shock syndrome secondary to a capillary leak phenomenon. Up to 500,000 such cases occur annually with approximately 25,000 deaths worldwide. There is currently no specific treatment available; however, some vaccine trials are in advanced stages.
Various factors have been implicated in the increased transmission of dengue virus of late. Warmer temperatures increase the length of time that a mosquito remains infective, and crowded conditions increase the potential for transmission. Global climate change with increased global temperatures is expected to further expand the range of A. aegypti and dengue virus.
Avian influenza H5N1 represents an emerging disease with currently focal distribution but with explosive potential, not unlike the 1918 global influenza pandemic that killed 20 million people. The 1918 pandemic caused by H1N1 remains, to date, the greatest infectious disease outbreak in history. This virus was an avian strain that adapted to infect and transmit among humans. Of the three influenza pandemics in the 20th century, two were caused by human-avian reassortment viruses (H2N2 in 1957 and H3N2 in 1968). Genetic reassortment between avian and human viruses leading to a new virus capable of pandemic spread may occur in coinfected persons or intermediate hosts like pigs that have receptors for both avian and human influenza viruses.
After its initial identification in May 1997 in a young boy who died of influenza in Hong Kong, sporadic transmission of avian influenza H5N1 to about 200 humans in Asia had raised concerns about an imminent pandemic.8 The virus is now endemic among bird and poultry populations in Eurasia and likely spread by migratory birds. There are concerns that the virus will adapt to a strain capable of sustained human-to-human transmission, unlike the current strain where almost all cases are secondary to direct contact with poultry.
Like most new influenza strains, the current H5N1 strain also emerged in Southeast Asia and rapidly spread westward, reaching Turkey by 2006. This spread has continued despite the culling of domestic and agriculture poultry flocks. Over 600 human infections have been confirmed in 15 countries including Thailand, Vietnam, Indonesia, Cambodia, China, Turkey, Azerbaijan, Egypt, and Iraq with a 50% case-fatality rate. Human cases from 2006 compared with 1997 show that the virus has undergone some antigenic changes (i.e., antigenic drift).
Clinical presentation may include symptoms related to both the respiratory and gastrointestinal systems. Most patients present with fever, upper respiratory symptoms, diarrhea, and pneumonia as documented by chest radiographs. Laboratory abnormalities may include elevated aminotransferases and pancytopenia with complications and death resulting from respiratory failure and multiorgan dysfunction.
The H5N1 avian influenza virus is resistant to amantadine and rimantadine but susceptible to the neuraminidase inhibitors oseltamivir and zanamivir. The latter are most effective early in the course of illness. Unfortunately, strains with a high level of oseltamivir resistance have already been identified from a few Vietnamese patients. In addition to treating the patient, household contacts are recommended to receive postexposure prophylaxis with oseltamivir 75 mg daily for 7 to 10 days.
The WHO and governments across the world have actively prepared for a pandemic by investing in vaccine development, stockpiling medication, and developing grassroots plans to cater to large numbers of patients. As of September 2012, the WHO had retained the avian influenza pandemic alert at phase 3. The following is a description of the six phases of pandemic alert:
- Phase 1: Interpandemic phase; low risk of human cases
- Phase 2: New virus in animals but no human cases; high risk of human cases
- Phase 3: Pandemic alert; no or very limited human-to-human transmission
- Phase 4: Clusters of human cases suggesting increased adaptability of the virus; evidence of increased human-to-human transmission
- Phase 5: Larger clusters of human cases over longer periods; evidence of significant human-to-human transmission
- Phase 6: Pandemic; efficient and sustained human-to-human transmission
The West Nile virus, another flavivirus, suddenly emerged in North America in August 1999. It was first identified as an outbreak of viral encephalitis in New York with 62 cases and 7 deaths.9 The virus has since progressively spread westward, and 2005 saw activity detected in 48 states and the District of Columbia. An arbovirus extensively distributed throughout Africa, the Middle East, Europe, South Asia and Australia, the North American strain is believed to have been imported from the Middle East, likely through air travel. In the United States, the peak of the outbreak was in 2003 when all but three states in the continental United States reported a total of 9,862 cases, 2,866 with neuroinvasive disease. Like other arboviral encephalitides, peak incidence was seen in late summer or early fall. In summer 2012, there was another surge in infections with the state of Texas hit particularly hard. Viral transmission has also spread to Canada, Mexico, the Caribbean, and Central America.
Mosquitoes of the Culex genus are the primary vectors with birds as the primary amplifying hosts, maintaining a bird-mosquito-bird cycle. Humans are incidental hosts and, unlike dengue, not important for transmission. Most are asymptomatic or present with a nonspecific febrile illness with only 1 in 150 infections resulting in meningitis or encephalitis, especially among the elderly. No specific treatment exists.
West Nile virus transmission in the United States has subsequently also been described with blood transfusions and organ transplantation, the latter often with catastrophic results.
In addition to the natural emergence and reemergence of disease, some biologic agents have the potential to be used deliberately to inflict mass casualties. Among weapons of mass destruction, biologic weapons are more destructive and cheaper to produce than chemical weapons, and they may be as lethal as nuclear weapons. It is estimated that the aerosolized release of 100 kg of anthrax spores upwind of Washington, D.C., would result in between 130,000 and 3 million deaths, similar to a hydrogen bomb.10 In 2001, the United States experienced a bioterrorism attack using anthrax powder distributed via the postal system.11 Three cases of anthrax were confirmed in South Florida in October 2001 with 19 additional confirmed or suspected cases from New York City, New Jersey, Maryland, Pennsylvania, Virginia, Connecticut, and Washington, D.C. Eleven of these were cases of inhalational anthrax, most occurring in postal employees. The FBI investigation, code named Amerithrax, was formally concluded in February 2010 after the prime suspect took his own life before charges could be filed. This attack remains the worst biologic attack in US history.
Although the list of potential biologic agents is large, the Centers for Disease Control and Prevention (CDC) has identified a number of high-priority organisms as category A agents based on their potential for easy dissemination, person-to-person transmission, and ability to cause panic, social disruption, and high mortality. Category B agents are moderately easy to disseminate and cause moderate morbidity and low mortality; category C agents include emerging pathogens that could be engineered for mass dissemination in the future because of availability (Table 12-3). Preparedness and planning for a bioterrorism-related event involves strengthening existing systems for detection and response to naturally occurring epidemics, particularly the emerging and reemerging infections. Through strong epidemiologic training, development of a communications infrastructure, a network of diagnostic laboratories, and a respect for the threat of biologic terrorism, preparedness can be improved and the impact of epidemics, regardless of the origin, can be reduced.
Category A | Category B | Category C |
|---|---|---|
Variola major (smallpox) Bacillus anthracis (anthrax) Yersinia pestis (plague) Clostridium botulinum (botulism) Francisella tularensis (tularemia) Filoviruses (ebola, marburg) Arenaviruses (lassa, junin) | Coxiella burnetii (Q fever) Brucella spp. (brucellosis) Burkholderia mallei (glanders) Burkholderia pseudomallei (meliodiosis) Chlamydia psittaci (psittacosis) Rickettsia prowazeki (typhus) Alphaviruses (encephalitis viruses) Ricin toxin Clostridium perfringens toxin Staphylococcus enterotoxin B Salmonella spp. (salmonellosis) Shigella dysenteriae (shigellosis) E. coli O157:H7 (enterohemmorrhagic E. coli) Vibrio cholerae (cholera) Cryptosporidium parvum (cryptosporidiosis) | Nipah virus Hantavirus Congo-Crimean hemorrhagic fever (CCHF) virus Tick-borne encephalitis viruses Yellow fever Multidrug-resistant tuberculosis (MDR-TB) |
Global Response to the Problem
In response to the Institute of Medicine report, the CDC developed a strategic plan in 1994 that was subsequently updated in 1998.12 Four interdependent goals were identified: