Embryologic Origination
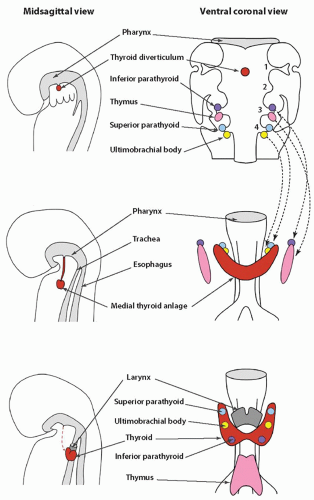
Knowledge of the embryologic development of the thyroid is key to understanding several abnormalities of the thyroid gland. The gland originates from the embryonic foregut, the same structure from which the pharynx, lungs, and upper digestive tract develop. The gland eventually contains two different types of hormonally active cells, follicular and C cells, and each type develops from a different embryologic structure. Follicular cells, which constitute the largest cell population, derive from the medial thyroid anlage. The C cells migrate to join the medial anlage through the lateral anlagen, also known as the ultimobranchial bodies (
Fig. 2.1).
Medial Thyroid Primordium
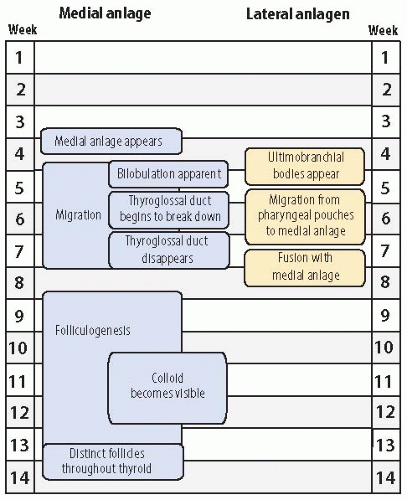
The medial primordium, or anlage, is first visible near the end of the third week of embryonic life (
Fig. 2.2). It appears as a thickening of endodermal epithelium of the cranial portion of the foregut, also known as the pharyngeal gut or pharynx.
1 The epithelial thickening develops in the ventral midline of the embryonic pharynx between the tuberculum impar and the copula (or hypobranchial eminence). This site, at the level of the second pharyngeal (or branchial) arch, eventually becomes the foramen cecum. The medial primordium soon invaginates to form a pit.
Migration
The invagination becomes more pronounced, yielding a flasklike diverticulum that begins to migrate caudally by the middle of the fourth week, concomitant with the descent of the heart. The migration of the thyroid anlage occurs through the loose mesenchyme ventral to the foregut. The cells continue to proliferate, and bilobation is evident early in the fifth week. The anlage, initially a hollow structure, solidifies with cells. It remains connected to the floor of the pharynx by the thyroglossal duct until the latter part of the fifth week when the duct begins to break down. The duct generally disappears between the sixth and eighth week, but portions may persist as fibrous cords or fine tubular structures with epithelial lining. Persistence of the latter into postnatal life can be the nidus of a thyroglossal duct cyst. As the medial anlage migrates, two lobes joined by an isthmus become visible during the sixth week. It reaches its final position anterior to the proximal region of the trachea during the seventh week of gestation.
Folliculogenesis
Formation of follicles begins in the 8th to 10th week as the thyroid transforms from solid cords of cells into rounded aggregates with small central lumina. Colloid is initially seen between the 10th and 12th week, and thyroid hormone is detectable in the fetal serum by the 11th to 12th week.
2,
3,
4 After the 13th week, the follicular cells appear morphologically well developed at the ultrastructural level,
4 and by the 14th week, distinct follicles are seen throughout the thyroid.
Growth and Maturation
The weight of the thyroid gland increases in a nearly linear manner from about 5 to 20 mg to 250 to 500 mg between the 10th and 20th week of gestation. The height of follicular cells stays between 12 to and 13
µm during the 10th to 20th week.
5 During this period of active folliculogenesis, the number of follicles per unit area initially increases and then declines, reflecting an increase in follicle size and colloid content. The follicular epithelium to colloid ratio declines until the 17th to 18th week. This ratio then remains fairly constant up to about the 29th week, suggesting the attainment of structural maturity early in the second trimester.
5,
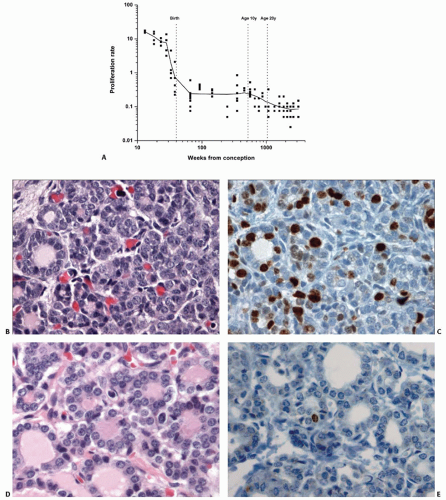
6 Proliferative activity in the thyroid as measured by Ki-67 (MIB-1) immunostaining of nuclei is relatively high between the 10th and 20th week, with averages ranging between 12% and 16% (
Fig. 2.3).
7 The rate diminishes throughout the latter half of gestation, dropping to <0.5% at the time of birth. The average proliferative rate remains a fraction of a percentage throughout the pediatric and adult periods of life.
The thyroid increases in size throughout gestation, showing the highest relative rate of growth during the second trimester.
8 During the second trimester, the gland weight increases 7- to 8-fold, increasing from approximately 100 to 125 mg to 700 to 800 mg.
5,
6,
9 Follicular cell height increases to reach a maximum of 18
µm at about 30 weeks.
5 After the 29th week, fetal thyroids tend to show a gradual increase in the epithelial:colloid ratio as epithelial growth outpaces colloid accumulation. The size of follicles decreases and the number of follicles per unit area increases, reflective of the relatively small volume of colloid.
Thyroid glands of newborn full-term infants have a variable appearance in terms of colloid content. A significant number shows no appreciable colloid, and some consider this a physiologic response to labor.
10 Desquamation of follicular epithelium and aggregates of pyknotic nuclei can be seen, and some have attributed this to physiologic changes.
10 However, in most cases, these latter changes probably represent postmortem autolysis. At end of 40 weeks of gestation, the thyroid gland attains a weight in the range of 1 to 4 g.
5,
9
Lateral Thyroid Primordia
The ultimobranchial bodies constitute the lateral thyroid primordia that fuse with the medial anlage to form the complete thyroid gland (
Fig. 2.1). The ultimobranchial bodies populate the thyroid gland with C cells.
11 The precursors of the C cells have been considered derivatives of the neural crest. The neural crest is a transient tissue initially found at the junction of the neural groove and ectoderm. After the neural groove fuses to form the neural tube, the neural crest forms an intermediate zone between the surface ectoderm and neural tube.
The pleuripotent cells of the neural crest migrate extensively throughout the body and differentiate into a wide range of cells including sensory neurons, postganglionic autonomic neurons and Schwann cells of the peripheral nervous system, melanocytes, chondrocytes, and catecholamine-secreting cells of the adrenal medulla.
12Using chick-quail chimeras, Le Douarin and Le Lièvre demonstrated that avian C cells ultimately derive from the neural crest.
13 They engrafted sections of quail neural primordium into chick embryos and traced the migration of the quail cells, distinguishable by their large nucleoli. Quail cells subsequently proved to be the predominant component of the ultimobranchial bodies and formed the C cells.
The assumption that mammalian C cells derive from the neural crest has been challenged recently. The ultimobranchial bodies of birds and lower vertebrates do not fuse with the medial anlage. Avian ultimobranchial bodies are innervated structures containing C cells separate from the thyroid gland, in contrast to mammalian ultimobranchial bodies, which fuse with the medial thyroid and disperse C cells within the gland.
14 It is of note that a recent study of mice found that neural crest cells populate pharyngeal arches but not the pouches including the site of the ultimobranchial bodies.
15 These findings suggest the origin of murine C cells from the endodermal epithelium as opposed to the neural crest. However, at this time, the orthodox view remains that C cells ultimately derive from the neural crest.
The ultimobranchial bodies become apparent during the fourth to fifth week of gestation as stratified endodermal tissue in contact with the embryonic pharyngeal space.
16 The ultimobranchial bodies are located in the most caudal pharyngeal pouches. Because the fifth pouch is rudimentary in humans, controversy exists whether it should be considered a separate fifth pouch or a component of the fourth pharyngeal pouch. Thus, the region containing the ultimobranchial body is sometimes referred to as the fourth-fifth branchial pouch complex.
16 For practical purposes in this chapter, ultimobranchial bodies will be considered part of the fourth pouch, acknowledging that they arguably constitute separate fifth pouches.
Migration and Fusion
The ultimobranchial bodies separate from the pharyngeal pouches and migrate centrally to fuse with the medial thyroid usually by the seventh to eighth week of gestation. Fusion typically occurs in the middle or midsuperior regions of the lateral lobes. After fusion with the larger medial anlage, the ultimobranchial bodies undergo dissolution with dispersal of C cells into the surrounding follicular tissue. Portions of the ultimobranchial bodies may persist in fetal or postnatal thyroid glands as small cystic structures or solid cell nests.
16,
17,
18,
19