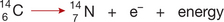chapter 1 Elements and compounds, chemistry and life
All physical objects and all compounds in our environment, both living and non-living, are made from matter. Matter can be defined as any substance which has mass and occupies space. This matter is made from the atoms of elements which are combined in a multitude of different ways to produce all the substances found in the environment.
Elements in living organisms
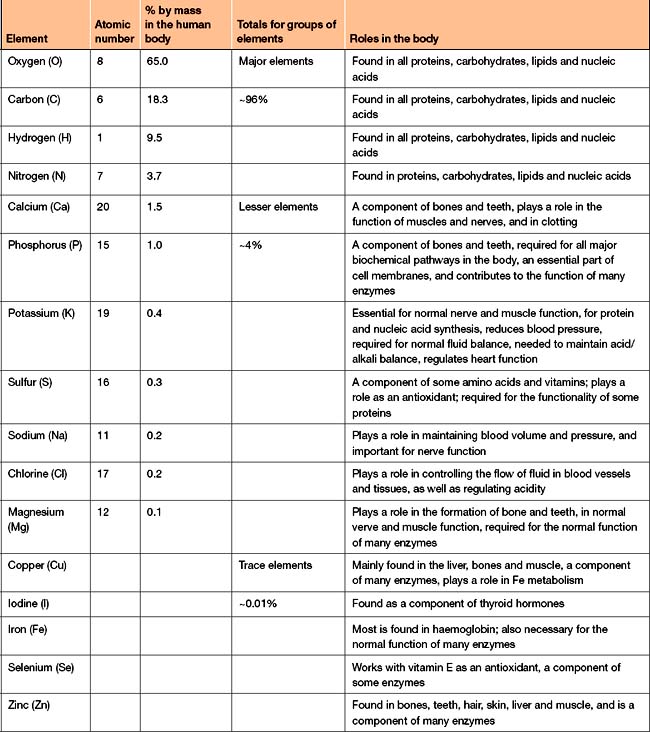
Only a small fraction of the naturally occurring elements are found in living matter. The major elements—carbon, oxygen, hydrogen and nitrogen—make up approximately 96% of the human body (Table 1-1). Seven lesser elements make up the remaining 4%. Other elements, referred to as trace elements, are required in very small amounts. For example, calcium, a lesser element, makes up about 1.5% of the human body whereas trace elements like selenium, copper and zinc make up less than 0.01% of the body’s mass.
Why are elements the way they are?
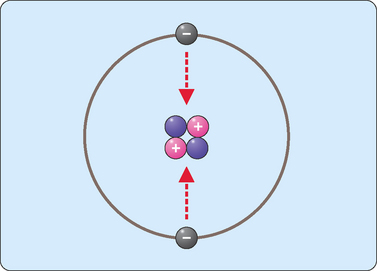
Each atom is composed of smaller components called subatomic particles which consist of neutrons, protons and electrons. Each proton has one unit of positive charge, an electron has one unit of negative charge, and a neutron, as its name suggests, does not carry a charge. Protons and neutrons are packed together at the centre of atoms to form the atomic nucleus. The electrons circulate around the nucleus, with the attraction between opposite charges keeping the electrons close to the nucleus (Fig 1-1). Neutrons and protons have roughly the same mass of about 1.7 × 10−24 g. However, such measurements are not very useful so we use a unit called a dalton instead. Thus, neutrons and protons are said to have a mass of 1 dalton (also called an atomic mass unit—amu). Electrons have negligible mass (1/2000th that of a proton/neutron) and are not used to calculate the total mass of an atom.