Fig. 5.1
Design of end friction machine
The pin was made of cartilages and the disc served as the counterbody. The machine was equipped with the system of friction torque measurement comprising induction gages and excluding the influence of angular and vertical displacements of the torsion device on the accuracy of friction force measurement (Fig. 5.2). The friction force, cartilage specimen deformation, and sliding velocity were recorded automatically.
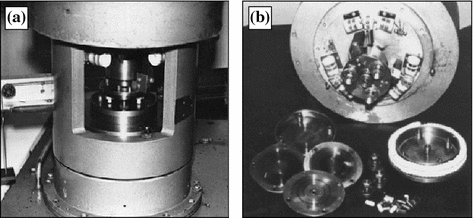

Fig. 5.2
Device for measuring friction force (a) and test specimens (b)
To improve the statistical significance of experimental data forty preliminary experiments were carried out with fresh synovia samples and cartilage specimens and the dependence of the friction coefficient on the load in the cartilage–glass pair was determined based on the averaged data. It was for reference in the subsequent selection of new cartilage specimens. For these specimens the similar dependence was determined and if the deviation between these and the reference data was statistically insignificant with the reliability of 99 % the specimens were used for tests with other lubricants. The experiments were repeated five times and their results were processed statistically.
Table 5.1 contains the results of the comparative tests of the cartilage–glass pair in different lubricants. The data show that the additive of rapic acid in the amount of 3 wt% into vaseline oil and water does not change the friction coefficient. This is caused by the inactivity of glass to fatty acids. According to the results of [9, 19], no stable adsorbed films of fatty acids appear on the glass surface. Therefore, under these conditions fatty acids are ineffective.
Table 5.1
Tribological characteristics of cartilage–glass (inactive surface) pair lubricated with synovia and model lubricants
Load (MPa) | Friction coefficient | ||||
|---|---|---|---|---|---|
Synovia | Water | Water + 3 w% of rapic acid | Vaseline oil | Vaseline oil + 3 wt% of rapic acid | |
0.2 | 0.015 | 0.35 | 0.35 | 0.05 | 0.05 |
0.6 | 0.009 | 0.25 | 0.20 | 0.04 | 0.035 |
1.0 | 0.007 | 0.20 | 0.20 | 0.03 | 0.025 |
1.5 | 0.008 | 0.18 | 0.18 | – | – |
2.0 | 0.009 | Unstable | Unstable | 0.025 | 0.025 |
3.0 | 0.010 | Unstable | Unstable | Unstable | Unstable |
4.0 | 0.010 | – | – | – | – |
5.0 | 0.012 | – | – | – | – |
6.0 | 0.013 | – | – | – | – |
The lubricant can also influence the formation of fatty acid adsorbed films. This influence consists in the concurring ability of molecules of the fluid base (water) in respect to fatty acid molecules [9]. As a result, water molecules react with the glass surface forming very stable silica gel films [10] which exclude the appearance of fatty acid adsorbed films on the surface.
Since in these experiments the introduction of rapic acid into the lubricants did not influence the friction coefficient and one of the friction surfaces was surely free of adsorbed fatty acid films we could suppose that other surface, i.e. cartilage one, showed a weak activity to fatty acids. Otherwise the friction coefficient should decrease because of interaction between fatty acid molecules and the cartilage surface forming stable boundary films on it [19]. This is confirmed by the study results of the friction of the cartilage specimen against active materials such as copper and steel 45 under lubrication with vaseline oil containing rapic acid (Table 5.2). Owing to the nature of these metals their surfaces can react physically and chemically with fatty acids which form adsorbed films of metallic soaps with a low shear resistance. This results in friction coefficient decrease.
Table 5.2
Tribological characteristics of cartilage–metal (active surfaces) pair lubricated with synovia and model lubricants
Friction pair | Load (MPa) | Friction coefficient | ||||
|---|---|---|---|---|---|---|
Synovia | Water | Water + 3 wt% of rapic acid | Vaseline oil | Vaseline oil + 3 wt% of rapic acid | ||
Cartilage–copper | 0.2 | 0.03 | 0.30 | 0.75 | 0.06 | 0.025 |
1.0 | 0.015 | 0.10 | 0.25 | 0.02 | 0.01 | |
2.0 | 0.01 | 0.08 | 0.17 | 0.015 | 0.01 | |
3.0 | 0.01 | 0.07 | 0.13 | 0.013 | 0.01 | |
4.0 | 0.013 | 0.05 | – | 0.01 | 0.008 | |
5.0 | 0.015 | 0.05 | – | 0.01 | 0.009 | |
6.0 | 0.02 | 0.05 | – | – | – | |
Cartilage–steel | 0.2 | 0.045 | 0.45 | 1.00 | 0.08 | 0.04 |
1.0 | 0.02 | 0.12 | 0.50 | 0.04 | 0.02 | |
2.0 | 0.01 | 0.07 | 0.30 | 0.03 | 0.01 | |
3.0 | 0.01 | 0.06 | 0.23 | 0.025 | 0.009 | |
4.0 | 0.01 | 0.05 | – | 0.02 | 0.008 | |
5.0 | 0.015 | 0.06 | – | 0.015 | 0.006 | |
6.0 | 0.02 | 0.05 | – | 0.015 | 0.006 | |
Along with this it should be noted that the introduction of rapic acid into water in friction of the cartilage against active surfaces induces more complex processes. In this case the friction coefficient increases 2–3 times compared with cartilage friction in pure water.
However, the results of model experiments with copper–copper and polymer–copper pairs lubricated with water containing rapic acid (Fig. 5.3, curves 1–4) show the rapic acid additive to reduce the friction coefficient. This proves a higher adsorption capacity of rapic acid molecules compared with water molecules that favors the appearance of adsorbed fatty acid films on the surfaces.
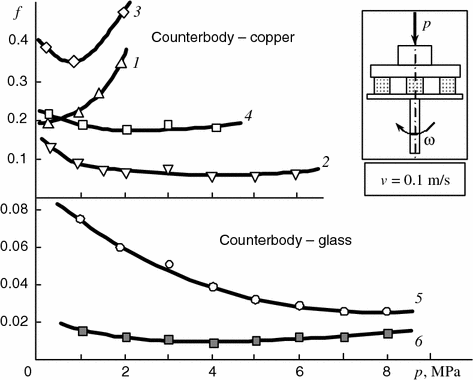

Fig. 5.3
Dependencies of friction coefficient of pairs copper–copper (1, 2), polyamide 6 copper (3, 4), cartilage–glass (5), and copper–glass (6) on pressure when lubricating with: 1, 3 distilled water; 2, 4 distilled water + 3 wt% of rapic acid; 5, 6 rapic acid
Therefore, when the cartilage rubs against active surfaces it contacts fatty acid lubricating films. Yet, as the results show (Table 5.2) such contact causes the friction coefficient to rise rather than decrease that is confirmed by the data on the friction of the cartilage–glass pair in pure rapic acid (Fig. 5.3, curve 5).
The above regularities can be explained in the following manner.
Since rapic acid molecules have a higher adsorption capacity compared with water molecules fatty acid boundary films are formed on the metal surfaces. The cartilage rubbing against the metal surface contacts the films. The cartilage has a porous structure [8, 21] with pores whose dimensions are comparable with those of rapic acid molecules. Therefore, fatty acid molecules penetrate into the pores and loosen them. This makes the wear of the cartilage surface layers severer and increases the friction coefficient due to the occurrence of the internal Rehbinder effect [18]. This mechanism is proven by the examination of the cartilage surfaces by scanning electron microscopy.
It has been found that in friction of cartilages against active surfaces in water containing rapic acid the structure anisotropy of the cartilage surface layers disappears (Fig. 5.4a, b). According to the data reported in [8, 22, 23] the thickness of the cartilage surface layer showing marked anisotropy is 200–600 μm. Therefore, anisotropy disappearance confirms certainly the catastrophic wear of cartilages in this case. In friction of the cartilage against the inactive surface with the same lubricants surface layer anisotropy does not disappear but wear particles are formed (Figs. 5.4c, d and 5.5a–f).
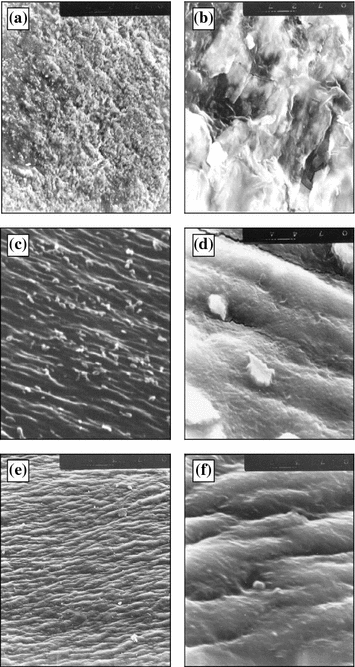
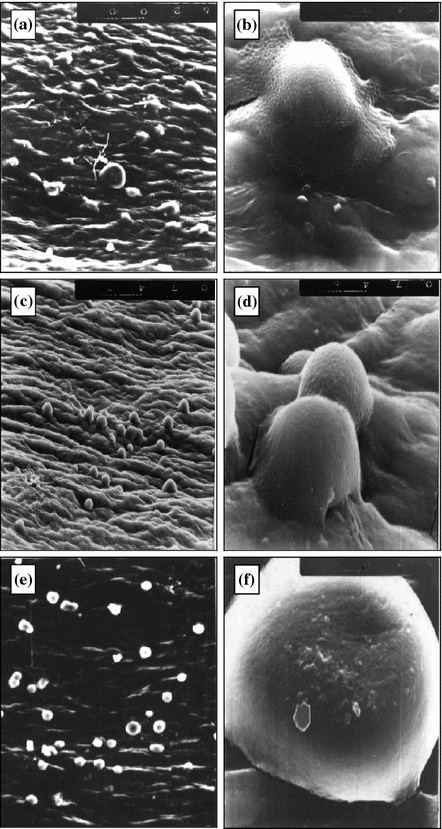

Fig. 5.4
Cartilage surface after friction against copper (a, b) and glass (c–f) in presence of: a, b distilled water + 3 wt% of rapic acid; c, d distilled water; e, f synovia. 270 × 330 μm (a, c, e); 32 × 40 μm (b, d, f)

Fig. 5.5
Kinetics of formation of spherical wear particles in friction of cartilage against glass in distilled water + 3 wt% of rapic acid. 270 × 330 μm (a, c, e); 32 × 40 μm (b, d, f)
The friction of the cartilage against glass in water causes flake-shaped particles to appear (Fig. 5.4c, d) and when adding rapic acid spherical particles have been found (Fig. 5.4a–f).
The observed wear of the cartilages in friction is apparently induced by the adsorption-fatigue damage of their surface layers typical for elastic materials [19, 23]. Different shapes of wear particles are explained by different effects of rapic acid and water on the cartilage surfaces. The introduction of rapic acid into water can make the plasticization of the cartilage surface layers severer compared with pure water and the material elongates at single sites (Fig. 5.5a, b). As friction continues, these sites experience repeated alternating deformations favoring the accumulation of damages (Fig. 5.5c, d) and the origination of microcracks followed by their merging and the appearance of spherical wear particles (Fig. 5.5e, f). However, in case of synovia consisting mainly of water (94 %) the friction coefficient for the active and inactive materials almost did not change (Tables 5.1 and 5.2) and no wear particles appeared (Fig. 5.4e, f). These results prove that synovia does not possess the properties typical for fatty acids.
It was believed for a long time that one of mucopolysaccharides, i.e. hyaluronic acid (HA), is the main lubricating component of synovia [8, 24, 25]. It has the structure typical for chiral biopolymers. Water solutions of HA show optical activity [26]. Solutions of such polysaccharides as cellulose and its derivatives whose structure is similar to that of HA have the mesophase [26]. Finally, the lubricating behavior of HA including the ability of changing concentration, flowability etc. proves the relation between the structure and functions of this biopolymer. All this indicates that HA may play a role in producing the mesomorphic state of the joint lubricant. Yet, the fact that the selective enzymatic depolymerization of HA does not affect significantly the lubricity of synovia [8, 27] requires the latter to be studied more thoroughly to find its other components capable of forming lubricating films with a low shear resistance and high load-bearing capacity.
From this viewpoint the ability of liquid crystal molecules to orient molecules of dissolved substances parallel to their major axes is of great interest for understanding the specific features of the lubricating behavior of joint synovia. This effect is called “guest–host” [11]. In paper [20] the influence of low-molecular liquid crystal additives on properties of the chiral polymers similar to HA was studied. Among substances capable of producing this effect esters of cholesterol and fatty acids being cholesteric liquid crystals are of primary interest. They belong to steroids and lipids which are widespread in biological structures. Therefore, it is natural to suppose that these compounds are contained in joint synovia and can play a significant role in the formation of its liquid crystal state [28, 29].
5.2 Synovia as Liquid Crystal Biological Fluid
It is known that esters of cholesterol and fatty acids presents in blood plasma in the liquid crystal state [30]. Cholesterol esters prevent arteries against damages due to blood pressure [31]. Since synovia originates from blood plasma they have similar compositions (Table 1.5). Yet, no data on the presence of cholesterol and its liquid crystal compounds in synovia are reported in publications and an experimental attempt to find them was made [29, 32].
The microstructure and chemical analyses of synovia were carried out in 2 h after the samples were taken [33].
The presence of cholesterol and its compounds in synovia was determined by the accelerated Ilka’s method based on the Liberman-Byrxard reaction [32]. Dried synovia samples were examined by polarization microscopy within the 20–50 °C range using a warm table. To exclude synovia decomposition the samples were dried by storing them for a long time (over 10 days and nights) at a negative temperature.
Synovia was taken from knee joints of fifteen animals and forty six patients (twenty eight men and eighteen women 11–80 years old) including twenty patients with rheumatic arthritis, eight patients with osteoarthrosis deformans, fourteen patients with traumatic synovitis, two patients with psoriatic arthritis, and two patients with infectious gonitis. Experiments were repeated two or three times for each sample.
The experiments have shown that the total content of cholesterol is 1.0–0.2 mmol/l (0.04–0.06 wt%) in animal and 3.8–0.4 mmol/l (0.16–0.20 wt%) in human synovia. The varying concentration of cholesterol in human synovia is apparently due to both the different character of joint pathology and age and individual peculiarities of the patients under study. However, no valid dependence of these changes on the nosologic form of the diseases was found.
The Ilka’s method is known to be capable of determining only the total content of cholesterol irrespective of the fact if the latter presents in synovia in the free or bound state. To obtain more accurate lipid composition of synovia the lipid fraction of sixteen synovia samples was examined by thin-film chromatography. Synovia was taken from three patients with rheumatic, four patients with traumatic, and nine patients with pigmented villonodular synovitis [20, 34]. The chromatograms of the synovia of the patients with pigmented villonodular and traumatic synovitis are shown in Fig. 5.6.
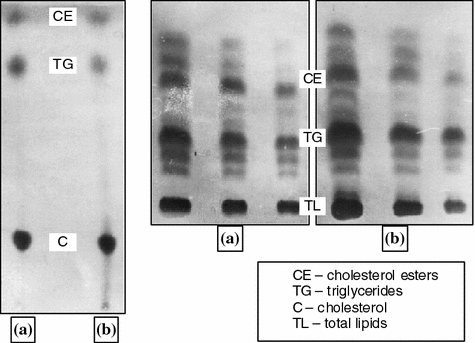

Fig. 5.6
Chromatograms of synovial fluids: a pigmented villonodular synovitis; b traumatic synovitis
The studies have shown that synovia contains cholesterol esters . Free cholesterol precipitated due to the addition of digitonin and it was found that the concentration of the esters in total cholesterol is 25–35 wt%.
Since cholesterol esters possess optical activity ten randomly taken synovia samples were examined by a polarizing microscope.
The tests have shown that dried human and animal synovia contains optically active inclusions. If the polarizers are crossed the inclusions are seen as luminous points or regions changing their color and the transmitted light intensity when rotating the sample. The thorough study has shown that single luminous points are radial aggregates resembling spherical crystallites found by S.S. Khalatov in blood plasma [35].
When the samples were heated to the temperature of 40–43 °C the inclusions became dark due to the transition of the liquid crystal phase to the isotropic fluid. The subsequent cooling of the samples to similar temperatures resulted to the appearance and retaining of the luminescence at lower temperatures because of the transition of the isotropic liquid back to the liquid crystal phase.
Figure 5.7 shows a luminous inclusion located near the crack edge appeared in the sample in drying. The heating of the sample to 24–26 °C causes the inclusion material to melt and spread over the crack that is accompanied by luminescence near the crack edges. When elevating temperature above 43 °C the luminescence disappears and it appears again when cooling the sample down to 40–43 °C. Subsequent cycles of heating and cooling result in the disappearance and appearance of the luminescence, respectively. Such behavior of the substance showing the luminescence has lead us to a conclusion that it possesses optical activity within the temperature range 25–41 °C and demonstrates features of a thermotropic liquid crystal compound.
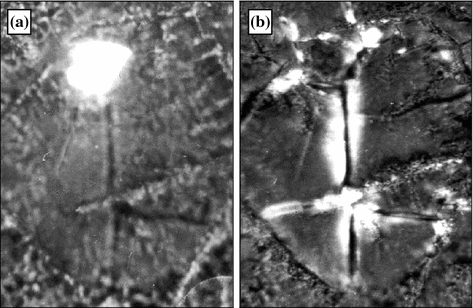

Fig. 5.7
Optically active elements of dried synovia before (a) and after (b) heating. 240 × 330 μm. Crossed polaroids
Therefore, the presence of cholesterol and its ester forms as well as a substance being mesomorphic at the human organism temperature in synovia prove that these compounds are cholesterol esters.
However, the methods used are incapable of identifying the compounds. The total fraction of cholesterol esters extracted from synovia by the chloroform-ethanol blend was examined by chromatography. The analysis has shown that synovia contains esters of cholesterol and the following acids (Table 5.3): palmitic (21.8–27.3 %), palmitic-rapic (1.0–4.3 %), stearic (7.9–13.1 %), rapic (21.1–25.8 %), linoleic (28.8–34.4 %), and arachidonic (5.2–9.5 %). As the data of Table 5.3 show, cholesterol esters of rapic, palmitic, and linoleic acids prevail in the composition of the total fraction of cholesterol esters.
Table 5.3
Composition of cholesterol esters identified in synovia of patients with arthritis of various aetiology (M ± m, %)
Cholesterol esters | Molecular weight, conventional units | Synovitis | ||
|---|---|---|---|---|
Pigmented villonodular (n = 9) | Rheumatoid (n = 3) | Traumatic (n = 4) | ||
Cholesterol palmitate | 624 | 25.1 ± 1.9 | 26.2 ± 1.1 | 24.1 ± 2.3 |
Cholesterol palmitoleate | 622 | 1.5 ± 0.5 | 2.1 ± 0.1 | 3.4 ± 0.9 |
Cholesterol stearate | 636 | 10.5 ± 2.6 | 9.1 ± 0.3 | 8.7 ± 0.8 |
Cholesterol oleate | 650 | 22.0 ± 0.9 | 23.6 ± 2.0 | 25.1 ± 0.7 |
Cholesterol linoleate | 649 | 32.0 ± 1.2 | 32.1 ± 1.9 | 31.6 ± 2.8 |
Cholesterol arachidonate | 672 | 8.9 ± 0.7 | 6.9 ± 0.3 | 7.1 ± 1.9 |
So, it has been shown that the natural joint lubricant contains the mixture of cholesterol esters which are known to be thermotropic liquid crystal compounds possessing the mesophase within a broad temperature range and having the cholesteric structure. Therefore, it is naturally to assume that a certain qualitative and quantitative combination of the esters produces a liquid crystal mixture with the mesophase within the physiological temperature range. Hence, one can expect that the joint lubricant itself possesses the liquid crystal state at the same temperatures.
The above assumption was verified experimentally [29, 36]. The occurrence of corresponding thermodynamic transitions in such systems is the most reliable criterion of belonging them to liquid crystal systems [28, 37]. For this reason the differential scanning calorimetry and probe fluorescence were used to estimate the temperature ranges of the mesophase of natural synovia and lipids extracted from it. The samples were taken from ten patients including five patients with rheumatic arthritis, two patients with pigmented villonodular synovitis, and three patients with traumatic synovitis.
The lipid fraction of synovia was extracted by the modified technique of Folch et al. [38]. Liposomes were obtained from the lipid suspension on an ultrasonic generator.
8-aniline-1-naphthyl sulfonate (ANS) served as the fluorescent probe. It is an amphiphilic molecule adsorbed well by lipid bi-layers with the orientation of charged sulfate units in the polar group plane. Since the quantum yield of ANS fluorescence depends on the surround polarity and can vary from 0.004 in water to 1.0 in non-polar media and the binding of ANS leads to the shift of the fluorescence maximum wavelength the probe fluorescence method is not only structure sensitive but also provides data on the molecular motion of some components of the liotropic biological fluid . In these experiments the 1 × 10−3 M water solution of ANS with the fluorescence maximum at 520 nm (the excitation wavelength was 360 nm) was used. The studies have shown that if ANS was added to synovia or liposomes (the final probe concentration was 1 × 10−5 M) its fluorescence maximum was registered at 485–490 nm (the excitation wavelength was 360 nm). This proves that ANS molecules are in the non-polar surround. The ANS fluorescence intensity reached its maximum at the following composition of the samples: 150–400 μl of synovia + 3.0 ml of the isotonic NaCl solution + 1 × 10−5 M of the ANS solution. The fluorescent analysis was performed on Jobin-Yvon and Fica instruments.
Figure 5.8 shows the results of the differential thermal analysis of synovia samples taken from patients with rheumatic arthritis (curve 1), traumatic (curve 2) and pigmented villonodular (curve 3) synovitis. The main phase transition in these biological systems occurs at 63–64 °C, the second transition—at 70–73 °C, and the third transition—at 86–88 °C. The transition temperatures vary within the 2–3 °C range depending on the nosologic form of joint pathology that is apparently due to the different content of water, cholesterol and other lipid components in the synovia samples under study.
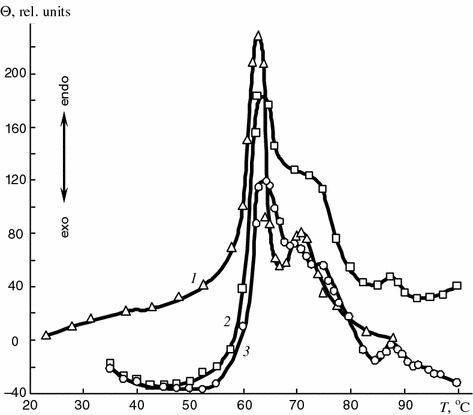

Fig. 5.8
Thermograms of synovial fluids of patients with rheumatoid (1) traumatic (2), and pigmented villonodular (3) synovitis
This assumption is confirmed by the following facts. In addition to hydrocarbon groups not reacting with water (lipophilic) lipid molecules contain one or more polar hydrophilic groups easily reacting with water. Such molecules are called amphiphilic to emphasize their dual nature. Owing to this dual nature and the selective interactions of two and more types of molecules the lyotropic mesomorphic phase may appear [1, 2]. Water solutions of cholesterol and its compounds in the presence of phospholipids are the examples of these systems [1, 38].
Lyotropic liquid crystal systems, like thermotropic ones, are characterized by a very high degree of molecular structure organization. They are very sensitive to changes in the temperature and concentration, e.g. water, cholesterol etc. [1, 2]. Variations in these characteristics induce phase transitions in the lyotropic systems accompanied by structure disordering. The high temperature of the transition in natural synovia (Fig. 5.8) allows one to assume that at these temperatures (63–65 and 70–73 °C) the phase transition of synovia components like lyotropic liquid crystal—isotropic fluid occurs. The third endothermic peak at 86–88 °C is apparently caused by the melting of desoxyribose nucleic acid which may appear in synovia due to cell disintegration.
To determine thermal transitions within the physiologic temperature range lipids of synovia were studied. Lipids extracted from synovia taken from a patient with pigmented villonodular synovitis were heated and the endothermic peak at 28.8 °C was found (Fig. 5.9).
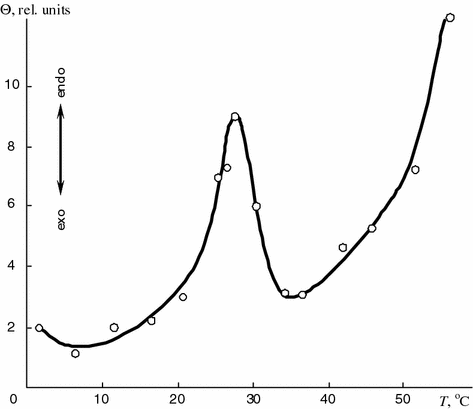

Fig. 5.9
Thermogram of lipids extracted from synovia of patient with pigmented villonodular synovitis
Since the reversible endothermic process occurred at that temperature it can be assumed that the latter is the temperature of the phase transition of the lipids from the crystal to mesomorphic state.
The comparison of the results obtained show that synovia components may have phase transitions within the range of physiological or higher temperatures that indicates the possibility of their liquid crystal state . It is also confirmed by the study results of synovia within a broad temperature range by the ANS fluorescence probe. The experiments have shown (Fig. 5.10) that the total temperature dependence of the intensity of the fluorescence of the ANS related to components of natural synovia has two inflections, i.e. at 31 and at 59 °C.
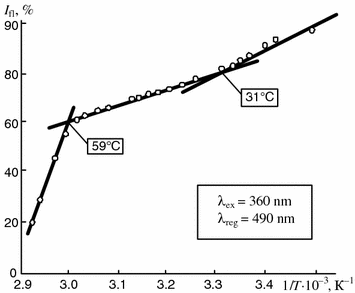

Fig. 5.10
Dependence of intensity of fluorescence of ANS bound to components of patient synovia on reverse temperature. The fluorescence intensity typical for tested samples at 20 °C is taken as 100 %
Figure 5.11 exemplifies individual temperature dependencies of the intensity of the fluorescence of the ANS related to components of natural synovia taken from patients with rheumatic arthritis and traumatic synovitis.
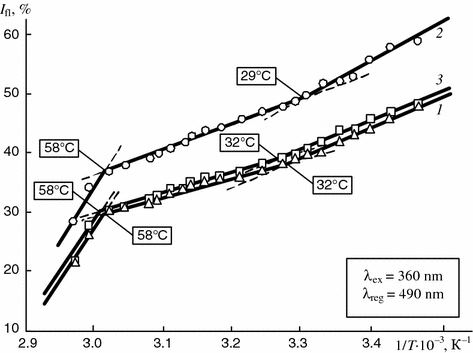

Fig. 5.11
Dependence of intensity of fluorescence of ANS bound to components of synovia of patients with rheumatoid (1, 3) and traumatic (2) synovitis on reverse temperature
The temperature dependence curves may show individual differences depending on the nosologic form of disease. The temperatures at which the inflections appear on the curves may wary by 2–3 °C (29–32 °C) within the physiological temperature range while at higher temperatures no variations occur and the ranges of temperature transitions for different diseases coincide exactly.
Than it was supposed that the inflections on the temperature dependencies of the natural synovia fluorescence intensity determined by using ANS result from the physical state, i.e. phase transitions, of lipids and cholesterol esters. To confirm this supposition, fluorescence characteristics of lipids and cholesterol fractions extracted from synovia were studied.
Figures 5.12 and 5.13 illustrate the temperature dependencies of the intensity of the fluorescence of the ANS related to the liposomes obtained from lipids and total cholesterol fractions.
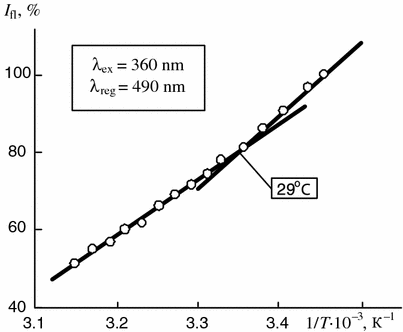
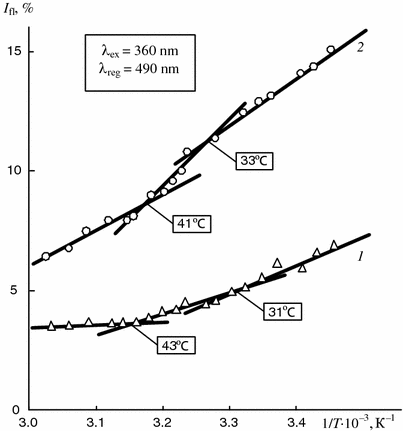

Fig. 5.12
Dependence of intensity of fluorescence of ANS bound to liposomes of total lipid fraction of synovia of patients with rheumatoid synovitis on reverse temperature (results of five experiments). The fluorescence intensity typical for tested samples at 20 °C is taken as 100 %

Fig. 5.13
Dependence of intensity of fluorescence of ANS bound to total cholesterol fraction of synovia of patients with rheumatoid (1) and pigmented villonodular (2) synovitis on reverse temperature
The analysis of the experimental data shows that the temperatures of the first phase transition determined for the lipid fraction of synovia by the differential scanning calorimetry (Fig. 5.9) are the same as the temperatures of the inflections on the temperature dependencies of the intensity of the fluorescence of the ANS related to both natural synovia (Fig. 5.11) and liposomes obtained from synovia lipids (Fig. 5.12) as well as to total cholesterol fractions (Fig. 5.13). This proves that the transitions result from changes in the physical state of one and the same biological substrates from the crystal to liquid crystal state.
Slight temperature variations observed for the specimens under study depending on the nosologic form of the disease can be explained by the fact that synovia is a dynamical system containing, in addition to water, phospholipids, and cholesterol, other components. The concentration of the latter can change due to the disturbance of metabolism in diseases, hence, it can influence the temperature transitions of the biological system as a whole.
At the same time two phase transitions occur within the physiological temperature range on the temperature dependence of the fluorescence of the ANS bound to the total cholesterol extracted from synovia. The first transition appears at 31–33 °C and the second one at 41–43 °C (Fig. 5.13). The first transition is typical for both lipids and total cholesterol fraction while the second one only for the latter fraction.
This fact can be explained only by phase transitions of the cholesterol compounds. Therefore, within the mentioned temperature range (41–43 °C) these compounds among them, according to the above chromatography data, cholesterol esters prevail undergo the phase transition similar to the liquid crystal—isotropic fluid transition. This conclusion is confirmed by the comparison of the obtained experimental data with the results of thermopolarization microscopic studies described above.
Thus, the combination of the results obtained by three independent methods (Table 5.4) proves that synovia has phase transitions at both physiological and higher temperatures. It should be noted that the temperatures determined for lipids of synovia by the differential scanning calorimetry practically coincide with the temperatures of the inflections on temperature dependencies of the fluorescence intensity of the ANS bound to both natural synovia and its total cholesterol fraction. The variability of the temperatures registered for these samples by different methods is within 2–3 °C. Therefore, the close temperatures determined for the synovia samples by the independent methods are due to changes in the structure state of one and the same biological objects.
Table 5.4
Temperature ranges of changes in physical state of natural synovia and components extracted from it
Study techniques | Phase transition | Temperature ranges of phase transitions of synovia and its components, °C | |||
|---|---|---|---|---|---|
Native synovia | Liposomes from lipids extracted from synovia | Lipids extracted from synovia | Total cholesterol fraction extracted from synovia | ||
Thermopolarization microscopy | I II | 25 41 | – | – | – |
Scanning differential microcalorimetry | I III IV V | 62–64 70–73 86–88 | – | 28–29 | – |
Probe fluorescence of ANS | I II III | 29–32 58–59 | 29 | – | 31–33
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|