Dysplasia in Barrett Esophagus
Elizabeth A. Montgomery, MD
Key Facts
Clinical Issues
No metastatic potential by itself
High-grade dysplasia (HGD)
In pooled data about 30% of patients with HGD progress to invasive adenocarcinoma
Low-grade dysplasia (LGD)
Regression to no dysplasia in about 2/3
Persistent LGD in about 20%
Progression to HGD/cancer in 13-15%
Microscopic Pathology
IFD: Uncertain whether process is reparative or neoplastic
Inflammation/erosions often a factor
Often surface maturation is present
LGD: Slightly crowded glands
Reduced surface maturation
Nuclei at surface are larger than those of nondysplastic Barrett mucosa
HGD: No surface maturation
Lost nuclear polarity, including at surface
Top Differential Diagnoses
Low-grade dysplasia
Reactive changes
IFD category has obviated some problems with this distinction
High-grade dysplasia
Intramucosal carcinoma
Somewhat subjective
Nucleoli, intraluminal necrosis, and syncytial effacement of lamina propria are features of early invasion
TERMINOLOGY
Abbreviations
Indefinite for dysplasia (IFD)
Low-grade dysplasia (LGD)
High-grade dysplasia (HGD)
Synonyms
Columnar epithelial dysplasia
Definitions
Neoplastic change confined to gland in which it arose
CLINICAL ISSUES
Presentation
May have reflux symptoms but often asymptomatic
Treatment
Options, risks, complications
Esophagectomy: Reserved for HGD
Standard in 1990s but has fallen from favor with advent of newer techniques
High morbidity and mortality, especially in low-volume centers
Higher initial costs and results in more frequent minor complications
Usually curative
Mucosal ablation methods (“endotherapy”)
Photodynamic therapy
Endoscopic endoluminal radiofrequency ablation (using the Barrx device)
Cryotherapy
Laser therapy
All of these endoscopic treatments can be complicated by strictures
Associated with higher risk of tumor progression, although still uncommon
Endoscopic mucosal resection
Prognosis
No metastatic potential by itself
Risk of progression to invasive carcinoma is key and informs suggested surveillance and treatment
In pooled data about 30% of patients with HGD progress to invasive adenocarcinoma
About 6 per 100 patient-years
In patients with LGD, regression to no dysplasia about 65%, persistent LGD in about 20%, and progression to HGD/cancer in 13-15%
Follow-up: Modified from American College of Gastroenterologists guidelines
Negative for dysplasia
Confirm with 2 EGDs with biopsy within 1 year
Follow-up endoscopy every 3 years
LGD (and IFD)
Confirm: Repeat EGD with biopsies within 6 months to ensure there is no HGD
Expert pathologist confirmation
Follow-up endoscopies at 1-year intervals until no dysplasia identified on 2 consecutive endoscopies
HGD
Repeat EGD with biopsies to rule out invasive carcinoma within 3 months
Expert pathologist confirmation
Endoscopic resection
Continued 3-month surveillance or intervention based on results and patient
MACROSCOPIC FEATURES
Routine Endoscopy
Dysplastic mucosa often indistinguishable from nondysplastic Barrett mucosa
MICROSCOPIC PATHOLOGY
Key Descriptors
Predominant pattern/injury type
Neoplastic
Predominant cell/compartment type
Epithelial, glandular
Histologic features
Neoplastic appearing nuclei
No invasive carcinoma
Indefinite for Dysplasia
Uncertain whether process is reparative or neoplastic
Inflammation/erosions often a factor
Some nuclear enlargement and hyperchromasia
Surface maturation is often present
Rare cases show “basal crypt dysplasia” pattern, but usually surface maturation is feature of repair
Normal to have some nuclear enlargement in deep glands of nondysplastic Barrett mucosa
Tangential embedding of such glands can be a factor and lead to overinterpretation of dysplasia
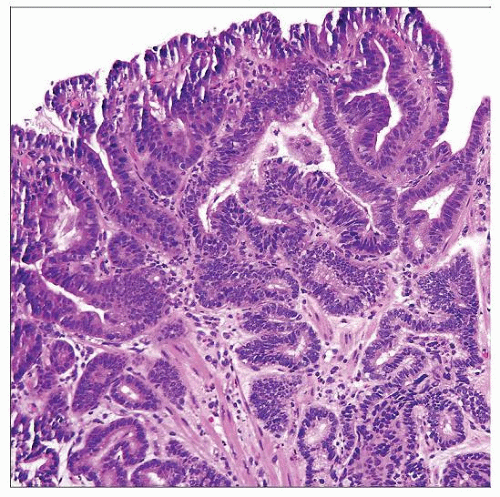
Low-Grade Dysplasia
Slightly crowded glands
“Upside down” goblet cells
Reduced surface maturation
Hyperchromatic nuclei at surface
Nuclei at surface are larger than those of nondysplastic Barrett mucosa
These features correlate well with image analysis findings
Image analysis not practical for clinical use but corroborates routine morphologic findings
Maintained nuclear polarity
Long axes of nuclei are perpendicular to basement membrane
Minimal nuclear membrane irregularity
Inflammation typically minimal
High-Grade Dysplasia
Crowded glands
No surface maturation
Lost nuclear polarity, including at surface
Nuclei have lost organized relationship to basement membrane
Nuclear membrane irregularities
More readily identified in material fixed in Bouin solution or Hollande fixative
Hyperchromatic nuclei
In most cases, internal control identified for comparison of nuclear hyperchromasia
Sometimes presents as nodule visible at endoscopy
Nucleoli not prominent
Occasional cases infiltrated by neutrophils
Possible influence of elaborated cytokines or chemotaxis factors
ANCILLARY TESTS
Immunohistochemistry
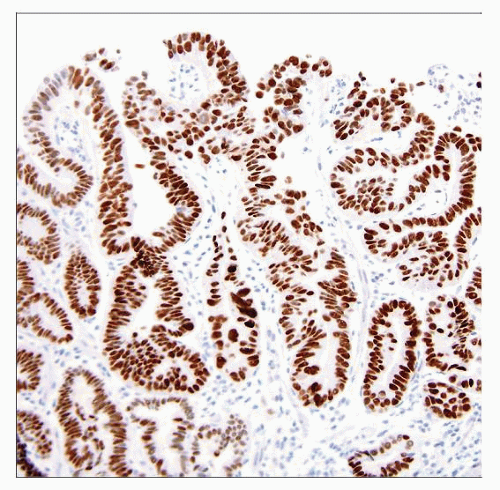
Usually not required for diagnosis, but many observers find p53 and Ki-67 labeling helpful
Caution: p53 labels about 90% of HGD
Subset is unlabeled
p53 in LGD has correlated with progression to HGD in some studies
Routine H&E remains best “marker” of progression to cancer
DIFFERENTIAL DIAGNOSIS
Low-Grade Dysplasia
High-Grade Dysplasia
Distinguish HGD from intramucosal carcinoma
Somewhat subjective
Nucleoli, intraluminal necrosis, and syncytial effacement of lamina propria are features of early intramucosal invasion
On endoscopic mucosal resection samples, immunohistochemical more easily distinguished from HGD
Duplicated muscularis mucosae can result in confusion about depth of invasion
Finding ulcers associated with HGD raises possibility of unsampled invasive carcinoma
SELECTED REFERENCES
1. Rastogi A et al: Incidence of esophageal adenocarcinoma in patients with Barrett’s esophagus and high-grade dysplasia: a meta-analysis. Gastrointest Endosc. 67(3):394-8, 2008
2. Schembre DB et al: Treatment of Barrett’s esophagus with early neoplasia: a comparison of endoscopic therapy and esophagectomy. Gastrointest Endosc. 67(4):595-601, 2008
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree