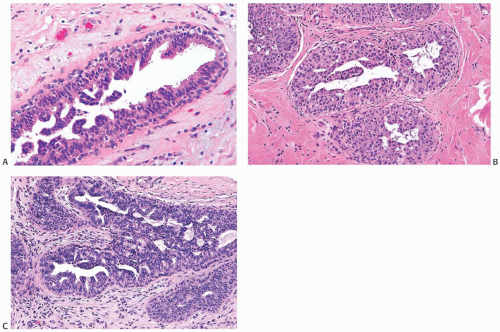
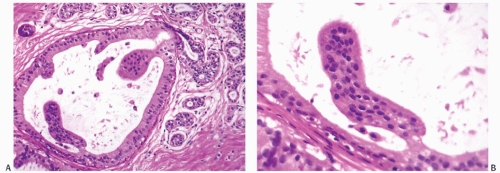
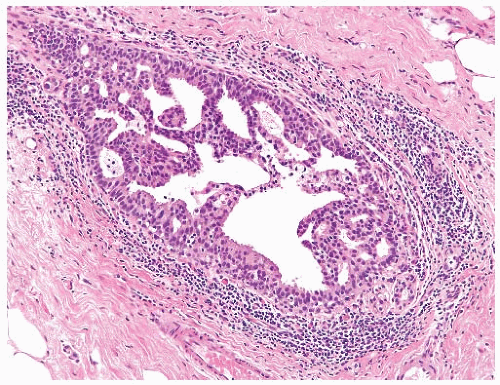
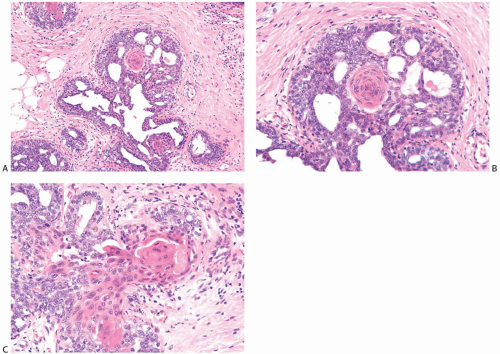
Ductal Hyperplasia: Usual and Atypical
SYED A. HODA
DEFINING DUCTAL HYPERPLASIA
This chapter is primarily concerned with the histopathology of various degrees and types of ductal hyperplasia. Chapter 10 addresses the concept of hyperplasia as a precancerous condition in greater detail. Chapter 11 is devoted to ductal carcinoma in situ (DCIS). Since these topics are intimately related, some subject matter may be covered in more than one chapter. The discussions of ductal hyperplasia and DCIS are presented in separate chapters to emphasize the importance of pathologically distinguishing between these entities in the clinical setting.
Ellis et al.1 proposed that epithelial hyperplasia of the breast be defined and “identified by a mixture of cell types demonstrated principally by morphology but that could be supported by immunophenotypic diversity and by lack of a dominant epithelial population.” It was further suggested that “the distinction of the boundary between hyperplasia and neoplasia in the breast should be recognized … [and] … is practicable at present…” with in situ carcinoma “identified by presence of a dominant clonal epithelial cell population that can be identified morphologically by a single cell population having distinctive morphologic, immunophenotypic and molecular genetic characteristics.” For research purposes, it may be useful to view the spectrum of intraductal proliferation as a continuum with no subdivisions. This concept is embodied in the terms mammary intraepithelial neoplasia (MIN)2 and ductal intraepithelial neoplasia (DIN).3 Although these alternative classifications replace the word carcinoma with neoplasia, they retain diagnostic groupings intended to distinguish lesions equivalent to hyperplasia, atypical hyperplasia, and DCIS of lower and higher grades. Boundaries are suggested for these groupings with no compelling evidence that they are more biologically or clinically meaningful than the existing classification or that they improve diagnostic reproducibility. The use of the term “neoplasia” for lesions that are generally agreed to be hyperplasia of the usual type (hyperplasia without atypia) is contradictory and confusing.
If ductal carcinoma in the breast evolves in a stepwise fashion comparable to that which has been proposed in other organs such as the colon,4 then it is likely that some stages in this progression are manifested in the histologic phenotype of intraductal proliferations. Conversely, some significant genotypical alterations may not be manifested in the histologic appearance of lesions. Proliferative foci grouped together under a diagnosis of hyperplasia might consist of genotypically diverse abnormalities with differing risks of carcinoma. The unraveling of this puzzle will be the main focus of research in the realm of “precancerous” breast pathology for years to come. Immunohistochemical and molecular analysis of microdissected samples will be necessary to identify relevant markers, and it is likely that studies based on such tests will eventually be incorporated into diagnostic procedures when the information becomes clinically useful and appropriate technology becomes available.
MOLECULAR CORRELATES OF DUCTAL HYPERPLASIA
Research to date on molecular correlates of usual hyperplasia, atypical hyperplasia, and “precancerous” breast pathology suggests that this is a complex and difficult undertaking. Genetic abnormalities have been detected by chromosomal analysis and by molecular techniques in lesions considered to be benign proliferative breast disease.5,6,7 These investigations have employed microdissection to isolate individual ductal lesions for molecular analysis. The results of studies that report loss of heterozygosity (LOH)6 or monoclonality8 in atypical ductal hyperplasia (ADH) must be interpreted with caution. Images purported by the investigators to show ADH in these two studies6,8 could be interpreted by others as DCIS. One may reasonably question whether molecular data should guide interpretation of histologic phenotype or if the reverse interpretation should apply. Nonetheless, it is apparent that molecular analysis is unlikely to resolve the diagnostic issues of interpreting “borderline” lesions in routine histologic sections in the foreseeable future. As long as the primary method for diagnosis is histologic examination, the information gained from molecular studies will become applicable to routine diagnosis only if it results in the identification of one or more specific cancer-associated markers and procedures are developed for studying LOH or other genetic alterations directly in situ in tissues.
Ultimately, correlation of molecular alterations in proliferative lesions with clinical outcome will be necessary to determine which phenotypical and genotypical changes are meaningful. Presently, these studies must be retrospective in the sense that the histologic samples to be analyzed
have been obtained sometime in the past and patient outcome is determined subsequently without standardized interval follow-up procedures. Prospective studies of the “natural history” of atypical proliferative lesions subjected to molecular analysis are likely to be difficult since the introduction of breast cancer chemoprevention with selective estrogen receptor (ER) modulators and other medications.
have been obtained sometime in the past and patient outcome is determined subsequently without standardized interval follow-up procedures. Prospective studies of the “natural history” of atypical proliferative lesions subjected to molecular analysis are likely to be difficult since the introduction of breast cancer chemoprevention with selective estrogen receptor (ER) modulators and other medications.
The complexity of the task in retrospective analysis is illustrated by several representative investigations. Kasami et al.9 studied microdissected samples of proliferative breast lesions from eight women. Follow-up ranging from 8 to 25 years was available for four patients. The samples were analyzed for LOH and microsatellite instability (MSI) at 10 loci. In one patient, five loci with MSI and two loci with LOH were found in a lesion described as a papilloma with florid hyperplasia and atypia, whereas 10 other proliferative lesions from this patient showed no genetic alterations or atypia. Three loci of MSI were detected in a proliferative lesion without atypia from another patient. Both patients were well more than 20 years after the biopsies that produced these samples. The authors concluded “that several genetic alterations in proliferative breast disease lesions may not indicate clinically meaningful premalignancy for remaining breast.” It is likely that large-scale studies of multiple proliferative lesions from substantial numbers of patients with well-defined follow-up will be needed to detect significant associations between genetic alterations and cancer risk.
One prospective investigation examined the relationship between human epidermal growth factor 2 (HER2) and p53 expression in benign breast lesions, and breast cancer risk in a nested case-control study.10 The patients were part of the Canadian National Breast Screening Study. Accumulation of p53 protein detected by immunohistochemistry in benign proliferative lesions was associated with an increased risk of developing carcinoma (adjusted odds ratio [OR], 2.5; 95% confidence interval [CI], 1.01 to 6.40). There was not an increased risk associated with HER2 overexpression (adjusted OR, 0.65; 95% CI, 0.27 to 1.53). However, p53 and HER2 are only rarely expressed in hyperplastic lesions, and when present they are usually associated with “borderline” abnormalities that are variously interpreted as atypical hyperplasia or in situ carcinoma. Mommers et al.11 studied the expression of several proliferation and apoptosis-related proteins in foci of usual ductal hyperplasia (UDH). Expression of HER2 and p53 were found in 2% and 8%, respectively, and decreased expression of Bcl-2 was detected in 16%. Other markers with abnormal expression in some examples of usual hyperplasia were cyclin D1, Ki67, p21, and p27. Twenty-three of 91 lesions (25%) expressed more than one abnormal proliferation or apoptosis-related protein.
Studies that report p53 accumulation detected by immunohistochemistry in hyperplastic lesions do not appear well supported by molecular analysis of p53 mutations in these lesions. Done et al.12 used microdissection to isolate foci of epithelial hyperplasia and DCIS associated with invasive carcinomas with known p53 mutations. The samples of DCIS had the same p53 mutations as the corresponding invasive carcinomas, but none of the hyperplasias exhibited any p53 mutations. The authors did not report immunohistochemical data for these specimens, but the results suggest that p53 mutations are not a frequent occurrence in hyperplastic lesions, even in a breast that harbors carcinoma with this genetic alteration.
Another complicating factor lies in the reported detection of genetic changes in histologically normal-appearing breast tissues. LOH has been described in cells in microdissected histologically normal lobules obtained from tissue adjacent to breast carcinomas.13 Gobbi et al.14 studied the immunohistochemical expression of transforming growth factor-β-receptor II (TGF-β-RII) as a breast cancer risk marker in hyperplastic breast lesions without atypia and in the normal breast. Reduced expression of TGF-β-RII in nonatypical hyperplasia and in adjacent lobules was associated with an increased risk of developing invasive carcinoma in this retrospective study. Expression of HER2 has also been detected in histologically normal breast tissue associated with breast carcinoma. Ratcliffe et al.15 used a combined in situ hybridization and immunohistochemical technique to assess this oncogene and its protein expression. Membrane staining was generally weak when present in normal breast tissue, corresponding to the level of expression in carcinomas lacking HER2 amplification. However, the presence of a signal detected by in situ hybridization in scattered nuclei in histologically normal cells may indicate an early stage of amplification preceding histologic evidence of transformation in isolated cells.
These findings raise concern about the specificity of associations between molecular markers and particular types of proliferative lesions. Some alterations may occur in tissues exhibiting little or no proliferative change. Were this to be a widespread phenomenon, then the criteria for selecting samples for analysis that currently concentrate on proliferative abnormalities might need to be revised. Ultimately, a combination of histology, immunohistochemistry, and molecular analysis may lead to a more clinically relevant classification of proliferative ductal lesions than the existing system based entirely on histologic criteria.
The distinction between intraductal hyperplasia and DCIS is obviously important for patient management.16 In most instances, intraductal proliferations are readily classified by pathologists on the basis of generally accepted histopathologic features as either ductal hyperplasia or in situ ductal carcinoma.17 There exists a subset for which assignment to either of these categories is less certain, and these abnormalities generally qualify as atypical hyperplasia. The existence of these “borderline” lesions, some of which may be diagnosed as hyperplasia or in situ carcinoma, depending upon which criteria are employed, is not a compelling reason for abandoning the existing practice of distinguishing pathologically and clinically between UDH, ADH, and DCIS. Studies of interobserver reproducibility in the diagnosis of highly selected examples of these lesions have focused undue attention on the diagnostic problem of “borderline” abnormalities that applies to a small percentage of proliferative breast changes.2,18,19
CLINICAL PRESENTATI ON OF DUCTAL HYPERPLASIA
There are no clinical features specifically associated with ductal hyperplasia. The alterations caused by epithelial proliferation in individual ducts or in multiple branches of a ductal system are typically microscopic in dimension and impalpable and may not harbor calcifications. It follows that specific hyperplastic processes are rarely the targeted lesion in needle core or excisional biopsies of breast. A mass lesion that incorporates elements of ductal hyperplasia may develop if there are coexisting stromal alterations such as pseudoangiomatous stromal hyperplasia (PASH) or if the process is associated with fibrocystic changes that may be detected clinically as a palpable mass or radiographically. In addition to ductal hyperplasia, the lesion complex can include one or more of the following pathologic changes that are discussed individually elsewhere in this book: sclerosing adenosis; cystic and papillary apocrine metaplasia; duct ectasia and associated inflammation; fibrosis, PASH; and lobular hyperplasia.
An important corollary to the lack of clinical indicators specific to ductal hyperplasia is an inability to determine the duration of these lesions. The date on which ductal hyperplasia was biopsied is customarily used as if it were the date of “onset” in follow-up studies. This practice, which is a consequence of inability to determine the preclinical duration of hyperplastic ductal lesions, could be a source of bias in assessing the precancerous significance of proliferative lesions in individual patients.
When clinically evident, ductal hyperplasia most often occurs in an ill-defined palpable area that is described as breast thickening. The nonspecific mammographic manifestations of these changes include altered ductal patterns, parenchymal distortion, nonpalpable mass lesions, calcification, and asymmetry. Calcifications are the most frequent mammographic indication of ADH in the absence of a palpable abnormality.20,21,22 Radial sclerosing lesions (RSLs) described on mammography as “radial scars” often have a component of ductal hyperplasia. Some RSLs contain microcalcifications. One of the most distinctive clinicopathologic forms of fibrocystic change with a prominent component of ductal hyperplasia is juvenile papillomatosis, which typically presents as a single, discrete, unilateral mass in women in their teens and early 20s23 (see Chapter 37).
When the indication for biopsy was a palpable abnormality in the era that preceded the widespread use of mammography, ductal hyperplasia was found in 25% or less of specimens obtained.24,25 Not more than 5% of these biopsies had ADH. The frequency of atypical abnormalities is somewhat higher among mammographically directed biopsies including surgical excisions and needle core biopsies.26,27 The yield of ADH in magnetic resonance imaging (MRI)-directed vacuum-assisted core biopsies ranges from 3% to 8% in several studies.28,29,30
Ductal hyperplasia can be found in female patients at virtually any age; however, these proliferative lesions are uncommon before puberty in women and in men at any age. In patients younger than 30 years, most examples of ductal hyperplasia occur either as juvenile papillomatosis23 or as one of the groups of lesions referred to as papillary duct hyperplasia in children and young women.31 The majority of women with ductal hyperplasia are between 35 and 60 years of age. A significant subset of these patients have hyperplasia of the columnar cell type, a lesion that is often multifocal and associated with microcalcifications.32,33 In a study of 10,032 women with benign breast diseases conducted at the Mayo Clinic, the mean age of UDH was 53.9 years and that of ADH was 57.8 years.34 Ductal hyperplasia becomes less frequent after age 60, and, when present, the growth pattern is usually less florid than in younger women. However, an occasional woman older than 60 years may be found to have extensive proliferative changes with florid ductal hyperplasia. This finding is likely to be accompanied by disproportionately less lobular atrophy than would be expected at this age, or there may be lobular hyperplasia with secretion in lobules. Use of exogenous estrogens can be documented in some of these cases. In general, factors that are associated with the development of breast carcinoma (outlined elsewhere in the book) are also associated with risk of the development of ductal hyperplastic lesions.35
GROSS PATHOLOGY OF DUCTAL HYPERPLASIA
There are no grossly apparent pathologic features specifically associated with intraductal hyperplasia. Specimen radiography, and its correlation with clinical mammography, is an important element in the evaluation of needle core and excisional biopsies performed for this group of diseases—primarily to ensure excision of the target lesion (usually calcifications) and to guide sampling for histology in larger specimens.
MICROSCOPIC PATHOLOGY OF UDH
Ductal hyperplasia describes a proliferative condition that is manifested histologically as an increase in the cellularity of ductal epithelium. Since the normal resting epithelium consists of a continuous monolayer of cuboidal to columnar epithelial cells supported by myoepithelial cells, an increase in the cellularity of this two-layer configuration constitutes hyperplasia. Physiologic hyperplasia during pregnancy is manifested by an increase in the number of glandular structures, as well as growth in the thickness of the epithelial layer, especially in lobular glands and ductules that constitute terminal duct lobular units (TDLUs). Concurrent but usually a lesser degree of expansion of the myoepithelium may be present.
Hyperplasia causes an increase in the thickness of the epithelial layer resulting in partial or complete obstruction of the ductal lumen at the site of the proliferative abnormality. If intraductal hyperplasia is traced in serial sections, it is often possible to observe the discontinuous, multifocal nature of the condition along the course of a single duct. Enlargement of the entire affected glandular structure is also frequently observed, in terms of both increased diameter and greater length, resulting in a sinuous structure. Various
distortions of the basic ductal architecture occur when hyperplastic ducts become more sinuous or they are incorporated into complex proliferative abnormalities such as papillomas or RSLs. Hyperplasia can extend into branches of a ductal system and frequently involves TDLU structures. Ductal hyperplasia has been described by names such as epitheliosis36 and papillomatosis.37 The former term was applied to “… the solid and quasi-solid benign epithelial proliferation which is found predominantly in small ducts, ductules and lobules.”36 Because it refers to epithelium in general, epitheliosis could be construed to include lobular hyperplasia. Intraductal hyperplasia includes a variable component of proliferative elements that contain some fibrovascular stroma; therefore, the distinction between epitheliosis and papillary hyperplasia is not always clear-cut. Consequently, there is no apparent advantage to replacing the term ductal hyperplasia with epitheliosis. Papillomatosis, often used interchangeably with ductal hyperplasia, is a term more appropriately applied to hyperplastic lesions in which a distinct fibrovascular structure supports papillary epithelial hyperplasia. Ductal hyperplasia that is not atypical is referred to as “usual,” “regular,” or “ordinary” to distinguish it from ADH.
distortions of the basic ductal architecture occur when hyperplastic ducts become more sinuous or they are incorporated into complex proliferative abnormalities such as papillomas or RSLs. Hyperplasia can extend into branches of a ductal system and frequently involves TDLU structures. Ductal hyperplasia has been described by names such as epitheliosis36 and papillomatosis.37 The former term was applied to “… the solid and quasi-solid benign epithelial proliferation which is found predominantly in small ducts, ductules and lobules.”36 Because it refers to epithelium in general, epitheliosis could be construed to include lobular hyperplasia. Intraductal hyperplasia includes a variable component of proliferative elements that contain some fibrovascular stroma; therefore, the distinction between epitheliosis and papillary hyperplasia is not always clear-cut. Consequently, there is no apparent advantage to replacing the term ductal hyperplasia with epitheliosis. Papillomatosis, often used interchangeably with ductal hyperplasia, is a term more appropriately applied to hyperplastic lesions in which a distinct fibrovascular structure supports papillary epithelial hyperplasia. Ductal hyperplasia that is not atypical is referred to as “usual,” “regular,” or “ordinary” to distinguish it from ADH.
Microanatomic Distribution of UDH
Instances of proliferative lesions originating in major lactiferous ducts or in larger terminal ducts can be readily found to document the capacity of the epithelium of these structures to undergo hyperplastic, atypical hyperplastic, or carcinomatous change. The term intraductal as it applies to hyperplasia or in situ carcinoma relates to a pattern of epithelial proliferation, as well as its principal site of anatomical distribution.
Studies of breast tissue by subgross dissection suggest that a subset of the proliferative changes commonly described as ductal hyperplasia arise from ductular structures of the lobules.38 The specific histologic features of hyperplasia arising in a major duct or in the components of an “unfolded” lobule are not readily distinguishable in conventional histologic sections, although the overall structure of the lesion and its microscopic distribution in the breast may suggest the anatomical level of origin.
When subgross dissection is performed, the breast tissue is fixed and processed by a method that makes it possible to study the specimen with a dissecting microscope. The tissue can thus be examined to visualize its three-dimensional structure and abnormalities in the ductal-lobular architecture. Foci of interest can be excised from subgross samples in the form of small tissue blocks and embedded for histologic examination. Microscopic study of these selected specimens has shown that lobular “unfolding” occurs as a result of dilation and stretching of TDLU structures. The dilated structures are typically termed ducts if they are more than three to four times the diameter of a lobular ductule. In some instances, the dilated structures remain clustered together, and there may be remnants of one or more lobules that have participated partially or not at all in the process of unfolding. The ducts formed in some unfolded lobules appear to drift apart so that their origin in a TDLU structure may no longer be apparent in two-dimensional histologic sections.
Whereas many of these dilated structures arising from unfolded TDLU structures maintain continuity with the ductal system, others appear to become isolated and cystic. Some cysts are formed from dilated acinar glands, representing the terminal portions of the intralobular duct system. Others seem to develop in segments of the ductal system, possibly caused by internal ductal obstruction due to hyperplasia or as a result of compression resulting from periductal fibrosis or proliferative changes. It appears that a frequent fate of unfolded TDLU structures is to form cysts or to be the site of hyperplastic changes (Fig. 9.1). This morphogenetic relationship is the probable explanation for the frequent coexistence of the pathologic alterations that constitute fibrocystic changes, including ductal hyperplasia.
Ductal hyperplasia may be limited to one or more isolated foci, or it may involve multiple contiguous foci in a segmental region of the breast. Occasionally, it appears to arise in more than one segmental ductal system. Quantitatively, the amount of hyperplasia in any one duct varies from a minimal increase in the number of cells to complete filling of the duct, with occlusion of the lumen. The size or degree of distension of a duct is not a direct function of the amount of epithelial hyperplasia at that site. Segments of ducts occluded by hyperplastic epithelium may have a relatively small diameter, and ducts that are dilated sometimes exhibit slight hyperplasia.
Cytopathology of UDH
The amount, growth patterns, and anatomical distribution of ductal hyperplasia vary greatly from one patient to another. The structural spectrum of ductal hyperplasia is heterogeneous. Nonetheless, there are certain features that these lesions have in common that are the basis for diagnosis.
The cytologic characteristics of UDH are similar, regardless of the amount of hyperplasia present. The cellular proliferation in ductal hyperplasia often has a syncytial appearance because individual cell borders are inconspicuous (Fig. 9.2). The cytoplasm is amphophilic or weakly eosinophilic and homogeneous. Cytoplasmic vacuolization may occur. True cytoplasmic microlumens that contain secretion that stains positively with the mucicarmine or Alcian blue-periodic acid-Schiff stains are exceedingly unusual in ductal hyperplasia. The presence of intracytoplasmic mucincontaining microlumens is an atypical feature that should occasion careful consideration of a diagnosis of DCIS or pagetoid lobular carcinoma in situ (LCIS). In making this determination, it is critical to distinguish between intracytoplasmic vacuoles and small spaces that are remnants of the central duct lumen caught between cells.
The cytoplasmic volume of hyperplastic ductal cells tends to be reduced by comparison with normal ductal cells, causing the nuclear cytoplasmic ratio to be elevated. However, there is little increase, if any, in nuclear size in UDH. Nuclei are round, ovoid to spindly or reniform, depending, in part, on the plane of section. Nuclear spacing is uneven, resulting in areas where the cells appear crowded and nuclei overlap.
Nuclear membranes are delicate, and the chromatin pattern is typically uniform. Clear nuclear vesicles that occur in some examples of ductal hyperplasia are intranuclear inclusions of cytoplasm. Some nuclei may display longitudinal grooves. Nucleoli are inapparent or inconspicuous unless there is apocrine metaplasia in the hyperplastic epithelium. Mitotic figures are infrequent, and when present they have a regular configuration.
Qualitative and Quantitative Aspects of UDH
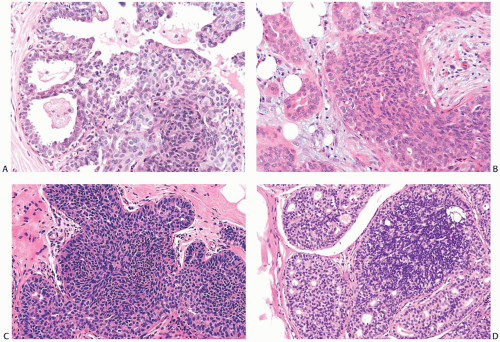
Ductal hyperplasia of the usual type has been subdivided on the basis of qualitative and quantitative criteria into the categories of mild, moderate, and severe or florid. The application of this classification is limited by the fact that disordered epithelial growth with varied structural patterns is a characteristic feature of ductal hyperplasia. As a consequence, hyperplastic epithelium is not uniformly distributed in a stratified fashion that permits easy determination of the number of cell layers. Epithelial thickness is also difficult to judge in tangentially sectioned ducts. Levels of hyperplasia based on epithelial thickness are most reliable when applied to selected nontangential sections of duct structures of sufficient diameter to manifest diagnostic features. Consequently, the classification of ductal hyperplasia based only on epithelial thickness has limitations and may not be applicable in all instances. Nonetheless, some degree of increase in the thickness of ductal epithelium is a characteristic feature of ductal hyperplasia.
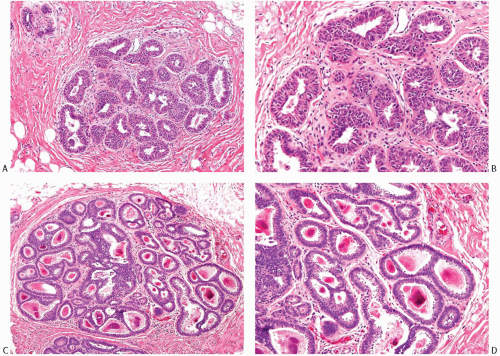
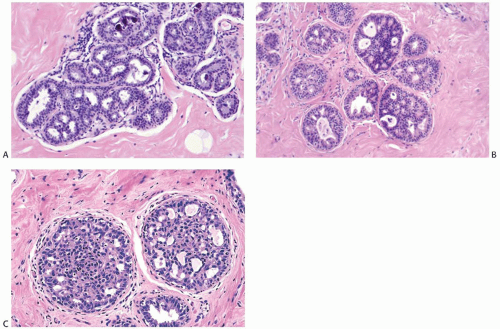
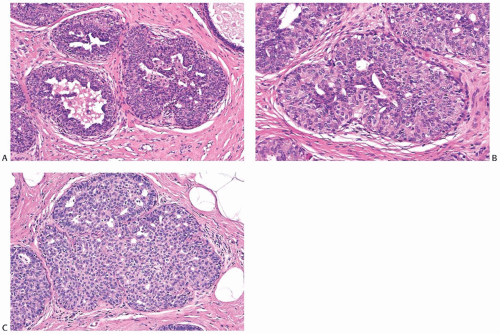
In mild ductal hyperplasia, the epithelium is three to four cells thick, exclusive of myoepithelium (Figs. 9.1 and 9.3). Mild hyperplasia may affect the entire epithelium circumferentially in a duct cross section, or only a segment of the duct. It usually occurs as a simple flat or slightly papillary increase in epithelial thickness. The diameter of the affected duct is generally not increased.
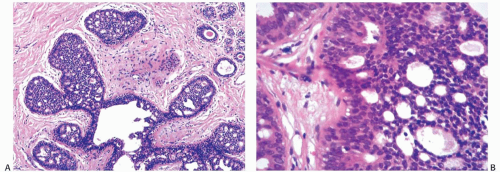
In moderate ductal hyperplasia, the epithelium consistently has a thickness of more than three cell layers. As in mild hyperplasia, the thickened epithelium may be distributed as a flat or papillary layer at the periphery of the duct. In some instances, relatively thin strands of epithelium extend across, or bridge, the lumen, resulting in the formation of secondary glandular lumina in the hyperplastic epithelium (Fig. 9.4). Part of the original ductal lumen usually remains as one or more crescentic spaces at the edge of the duct. The lesional cells around the secondary lumina tend to lie parallel rather than perpendicular to the long axis of the spaces. The diameter of ducts with moderate hyperplasia may be increased compared with unaffected ducts.
Micropapillary ductal hyperplasia is part of the spectrum of moderate ductal hyperplasia (Figs. 9.5 and 9.6). Micropapillary structures typically have a broad base and a narrow apex. The micropapillae are unevenly shaped fronds of hyperplastic epithelium in which the apical cells are smaller and have more condensed nuclei than those in the underlying basal epithelium or in the intervening nonpapillary basal epithelium (Fig. 9.7).
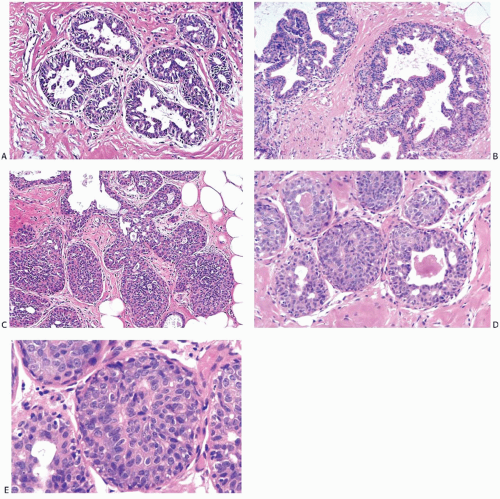
The distinction between moderate and marked or florid hyperplasia is not sharp. Lesions are generally placed in the latter category when the affected ducts are appreciably enlarged in comparison with nonhyperplastic counterparts and the lumina are nearly or completely filled by the proliferative epithelium. Squamous metaplasia sometimes develops in moderate and florid hyperplasia (Fig. 9.8). Squamous metaplasia may also be prominent in some cases of gynecomastia that exhibit micropapillary or florid duct hyperplasia. Moderate and severe ductal hyperplasia together constitute the category of ductal hyperplasia without atypia (“ordinary” or “usual” ductal hyperplasia) in the classification of proliferative breast changes used in the assessment of breast cancer risk discussed in Chapter 10.
The nuclei in moderate ductal hyperplasia are often overlapping and may be distributed in a “streaming” fashion. Streaming refers to a growth pattern in which hyperplastic epithelial cells are oriented parallel to their long axes, an appearance most readily appreciated in the distribution of nuclei (Fig. 9.9). Since the cytoplasmic borders of these cells are often indistinct, “streaming” is usually detected as a parallel orientation of oval- or spindle-shaped nuclei. The spectrum of streaming is broad, ranging from subtle foci composed of only a few cells to conspicuous “swirling”
patterns. Streaming occurs in all types of usual and atypical intraductal hyperplasia. The streaming and swirling of hyperplastic cells is reminiscent of a “school of fish” in appearance and is typically limited to the center of the involved duct space.
patterns. Streaming occurs in all types of usual and atypical intraductal hyperplasia. The streaming and swirling of hyperplastic cells is reminiscent of a “school of fish” in appearance and is typically limited to the center of the involved duct space.
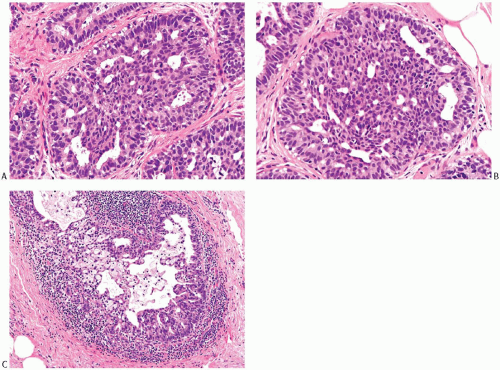
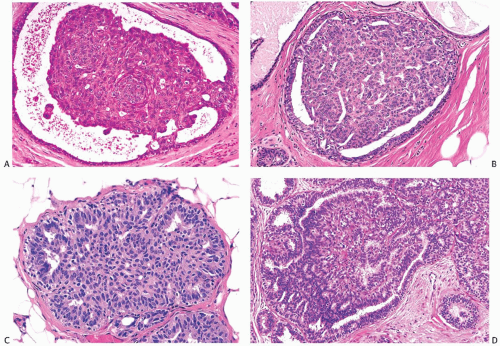
The distinction between moderate and florid hyperplasia is not sharp. Florid ductal hyperplasia has papillary and bridging growth patterns that are encountered in moderate hyperplasia, but the overall proliferation tends to be more cellular and complex than in moderate hyperplasia. Lesions are generally classified as florid when the affected ducts are appreciably enlarged in comparison with nonhyperplastic counterparts. Foci of florid hyperplasia are more likely to fill the entire duct lumen in a solid or fenestrated (cribriform) fashion. The cells are often distributed in a streaming pattern in solid or in fenestrated areas. The association of the streaming pattern with ductal hyperplasia has been confirmed by computerized morphometric analysis of the orientation of nuclei in proliferative ductal lesions.39 A part of the original ductal lumen may remain as a crescentic space or spaces at the edge of the duct (Figs. 9.10, 9.11, 9.12 to 9.13). The predominantly peripheral distribution of microlumens, also known as “secondary lumens,” that characterizes moderate and florid hyperplasia is an important difference from
cribriform DCIS, wherein microlumens tend to be distributed more evenly across the entire duct cross section.
cribriform DCIS, wherein microlumens tend to be distributed more evenly across the entire duct cross section.
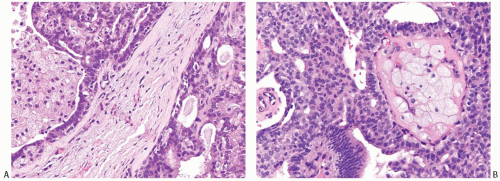
Necrotic cellular debris is rarely present in hyperplastic ducts, and when found it is usually associated with florid sclerosing papillary hyperplasia. This phenomenon is illustrated in Chapter 5. Hyperplastic ducts with necrosis are cytologically and structurally indistinguishable from adjacent ducts with nonnecrotic hyperplastic epithelium. A distinction should be made between necrosis and the accumulation of dense secretion admixed with minimal cellular detritus and inflammatory cells (Figs. 9.13 and 9.14). Histiocytes or foam cells are found relatively often in hyperplastic duct epithelium that lacks necrosis. The epithelium in florid sclerosing papillary ductal hyperplasia may have isolated mitotic figures as well as focal necrosis, but in this setting this combination is not by itself diagnostic of carcinoma.
The fenestrated (cribriform) growth pattern that occurs in moderate and florid ductal hyperplasia results from the
joining of epithelial bridges as they traverse the duct lumen. The fenestrations represent residual portions of the original ductal lumen that have been passively subdivided by the complex arborizing epithelial proliferation. Using a serialsection three-dimensional reconstruction method, Ohuchi et al.40 demonstrated that the lumens that appear to be separated from each other in a two-dimensional histologic section of intraductal hyperplasia are actually part of a network of channels surrounded by the proliferating epithelium. By contrast, three-dimensional reconstruction of DCIS revealed
that the fenestrations in these lesions were newly formed disconnected spaces bordered by polarized neoplastic cells.
joining of epithelial bridges as they traverse the duct lumen. The fenestrations represent residual portions of the original ductal lumen that have been passively subdivided by the complex arborizing epithelial proliferation. Using a serialsection three-dimensional reconstruction method, Ohuchi et al.40 demonstrated that the lumens that appear to be separated from each other in a two-dimensional histologic section of intraductal hyperplasia are actually part of a network of channels surrounded by the proliferating epithelium. By contrast, three-dimensional reconstruction of DCIS revealed
that the fenestrations in these lesions were newly formed disconnected spaces bordered by polarized neoplastic cells.
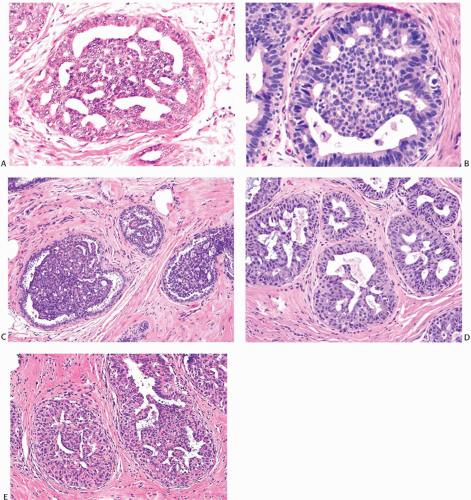
The spaces that are found in histologic sections of fenestrated intraductal hyperplasia have distinctive features. The secondary lumens tend to be larger and more numerous at the periphery of the duct than centrally, but variation of this distribution can be encountered (Figs. 9.4, 9.8, 9.11, and 9.12). Cells outlining these spaces are arranged in a haphazard fashion, except at the perimeter of the duct where residual columnar or cuboidal ductal epithelium composed of cells with oriented nuclei sometimes persists (Figs. 9.10 and 9.13). The spaces in a given hyperplastic duct usually have varied shapes rather than being rounded as they tend to be in cribriform carcinoma. In hyperplasia, the spaces may be ovoid, crescentic, irregular, or serpiginous, although in exceptional cases the lumens may be round and larger toward the center of the duct (Fig. 9.15). Cell membranes bordering on the spaces tend to be smooth, or they may present an uneven finely serrated surface that results from cytoplasmic blebs formed by complex folding of the cell membrane and microvilli41 (Fig. 9.16). Apical blebs that result in a fluffy cytoplasmic border resemble the “snouts” that characterize apocrine cells. In the absence of other apocrine cytologic features, apical cytoplasmic blebs should not be interpreted as a manifestation of apocrine change.
The spaces formed in intraductal hyperplasia usually appear to be devoid of cells and secretion. Occasionally, there may be histiocytes or lymphocytes present, sometimes with wisps of secretion. Calcification in the form of coarse granular concretions, calcospherites (calcification admixed with proteinaceous secretion), or crystalline deposits are uncommon in UDH unless there is an associated sclerosing component such as adenosis or a radial scar configuration. Columnar cell hyperplastic lesions are an exception to this generalization, since they are prone to form multifocal calcifications with distinctive histologic characteristics as described in subsequent text.
It is usually difficult to detect myoepithelial cells in ductal hyperplasia, except for the layer of these cells that is present at the edge of the duct. Myoepithelial cells may accompany the proliferation into the duct lumen when the fibrovascular stromal framework of solid papillary hyperplasia is present. Immunostains are useful for highlighting the myoepithelium in proliferative ductal lesions. The reactivity of individual myoepithelial markers is unpredictable in a given case, and it is advantageous to employ at least three markers.
p63, a member of the p53 family that is important in the development of epithelial tissues, is the only marker currently available that is localized exclusively in the nuclei of myoepithelial cells. It is not reactive with myofibroblasts or blood vessels.42 p63 is rarely reactive with scattered epithelial cell nuclei in papillary lesions and ductal hyperplasia. These epithelial cells can be distinguished from myoepithelial cells by their cytologic appearance or their position. Nuclear staining of myoepithelial cells typically produces a string of dots between the epithelium in ducts and lobules and the basement membrane.
Several cytoplasmic markers are available for highlighting the myoepithelium: smooth muscle actin (SMA), smooth muscle myosin-heavy chain (SMM-HC), calponin, CD10, CK5, CK14, and maspin. These markers exhibit variable cross-reactivity with epithelial cells, myofibroblasts, or blood vessels.43,44 Myofibroblastic reactivity is greatest with SMA or calponin and least with SMM-HC and CD10 (common acute lymphoblastic leukemia antigen [CALLA]).
However, many exceptions can be encountered. For this reason, as well as the variable reactivity of these reagents with myoepithelial cells, it is prudent to employ two or more cytoplasmic markers as well as p63 to evaluate the myoepithelium in a given case.
Myoepithelium is highlighted with immunostains in normal ducts and lobules. When proliferative fibrocystic changes occur in these structures, such as adenosis or ductal hyperplasia, the myoepithelium is usually uniformly present and it may be hyperplastic. Attenuation of myoepithelial cells, which occurs in some hyperplasias, especially sclerosing papillary lesions and ductal hyperplasia with atypia, results in increased space between p63 reactive nuclei compared with the staining pattern in normal structures. In this situation, the presence of the attenuated myoepithelium can usually be demonstrated with one of the cytoplasmic markers for these cells. Because of the stromal proliferation that accompanies many of these lesions, care must be taken not to mistake myofibroblastic reactivity for myoepithelium. Myoepithelium persists, admixed with the neoplastic epithelial proliferation, in some examples of DCIS, where it is usually attenuated. On the other hand, a pronounced degree of intraductal proliferation devoid of myoepithelium that is confirmed with immunostains, in the presence of internal positive controls, is very likely to be in situ carcinoma.
Collagenous spherulosis is an unusual finding in ductal hyperplasia and LCIS. In this condition, myoepithelial cells contribute to the formation of spherules of basement membrane material akin to those found in adenoid cystic carcinoma. The center of the spherule can become degenerated, so that it resembles a glandular lumen. Immunostains will demonstrate myoepithelial cells around spherules and distinguish these structures from coexisting true glandular lumina in cribriform hyperplasia and from the lumina
in cribriform DCIS. CD117 (c-kit) can be helpful in distinguishing collagenous spherulosis from adenoid cystic carcinoma. The epithelial components of collagenous spherulosis are ER (+), progesterone receptor (PR) (+), and CD117 (-), whereas its myoepithelial component is reactive for p63, CD10, myosin, etc.45
in cribriform DCIS. CD117 (c-kit) can be helpful in distinguishing collagenous spherulosis from adenoid cystic carcinoma. The epithelial components of collagenous spherulosis are ER (+), progesterone receptor (PR) (+), and CD117 (-), whereas its myoepithelial component is reactive for p63, CD10, myosin, etc.45
MICROSCOPIC PATHOLOGY OF ATYPICAL DUCTAL HYPERPLASIA
There is broad agreement on the general description of ADH as a proliferative lesion that fulfills some but not all criteria for a diagnosis of DCIS. By extension, it can be stated that
ADH has features of ordinary hyperplasia and of DCIS. The difficulty in arriving at a crisper definition lies in the specifics. In general, these can be considered under two headings: quantitative and qualitative. The former refers to the amount of a proliferative abnormality, whereas the latter is concerned with microscopic structural and cytologic details.
ADH has features of ordinary hyperplasia and of DCIS. The difficulty in arriving at a crisper definition lies in the specifics. In general, these can be considered under two headings: quantitative and qualitative. The former refers to the amount of a proliferative abnormality, whereas the latter is concerned with microscopic structural and cytologic details.
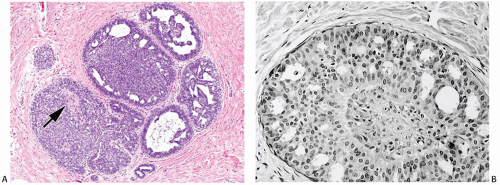
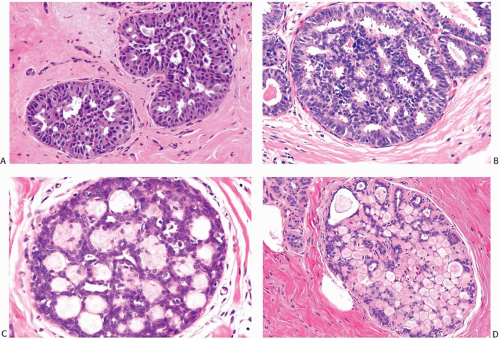 FIG. 9.16. Ductal hyperplasia, cribriform. A,B:
Get Clinical Tree app for offline access
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree


|