Drugs Used in Neoplastic Disorders
Overview
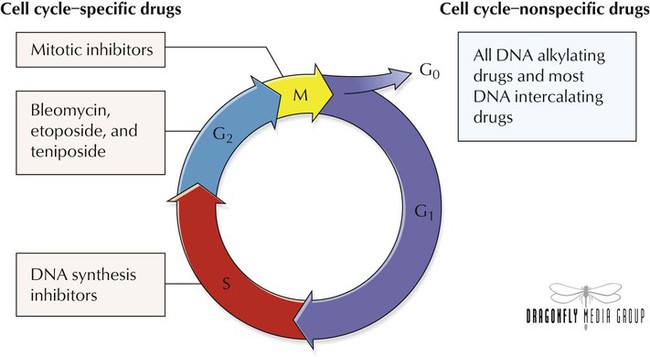
To replicate, both normal and cancer cells proceed through the cell cycle, which is divided into G0, G1, S, G2, and M (mitosis) phases. In the postmitotic G1 phase, cells produce many enzymes required for DNA synthesis. In the G0 phase, cells are resting but are still viable and can enter cell division. During S phase, DNA content doubles in preparation for cell division. In premitotic G2 phase, additional protein and RNA synthesis occurs. Antineoplastic agents cause cytotoxicity by affecting events occurring during these phases. Drugs that destroy cells only during a certain phase are cell cycle specific; cell cycle–nonspecific agents destroy cells independently of the phases. Chemotherapy is most effective against replicating tumor cells. Cell cycle–nonspecific agents, however, can be useful against tumors with few replicating cells. Chemotherapy is given intravenously, orally, intramuscularly, or subcutaneously or as a bolus injection, a short infusion, or a continuous infusion.
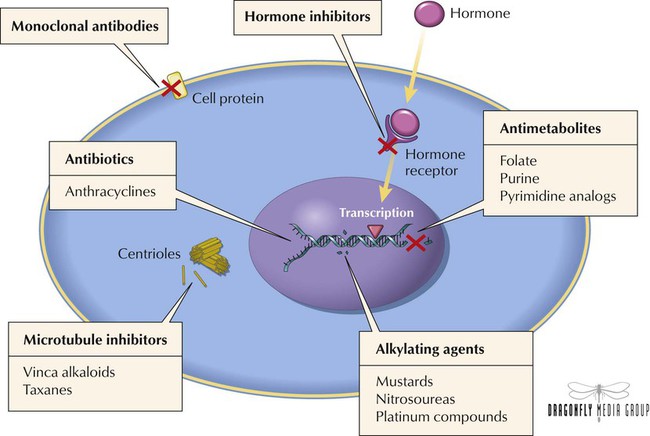
Aside from a few hematologic malignancies, most tumors show only a partial, fleeting response to monotherapy. Combination chemotherapy provides higher and more durable response rates by displaying efficacy against a broader range of cell lines in heterogeneous tumors, preventing or slowing development of resistance, and providing maximal cell kill (a measure of the number of tumor cells killed by drugs in relation to dose). Combination chemotherapy has thus become the standard for most malignancies. Selection of agents for regimens is based on the following principles: Only agents with demonstrated activity as monotherapy against the specific type of tumor should be selected. All agents within the regimen should have different mechanisms of action (which often has additive or synergistic effects). To minimize unacceptable toxicity, agents should not have overlapping adverse effects. To optimize efficacy and minimize resistance, the optimal dose and schedule of the drugs should be used.
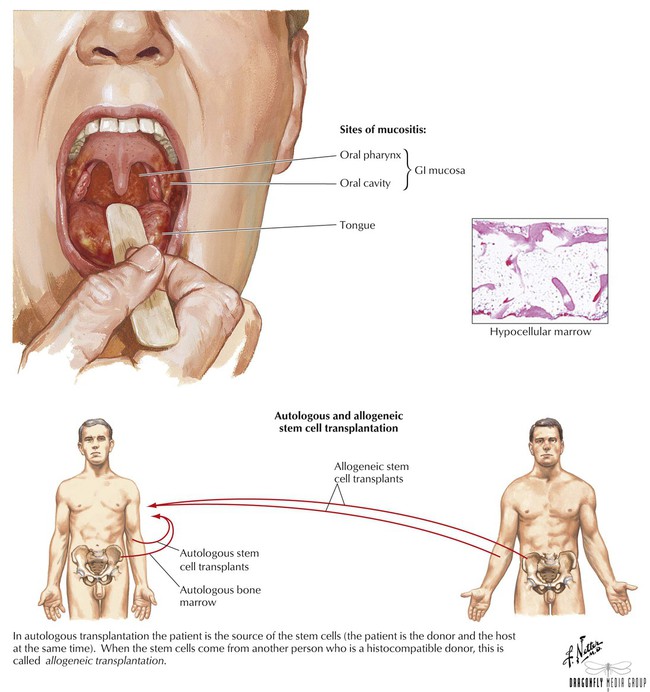
Most agents, especially older ones, do not discriminate between normal and abnormal cells and thus affect all proliferating cells, including those found in bone marrow, buccal and GI mucosa, and hair follicles. This nonselective feature helps to explain toxicities associated with these drugs. To some extent, most such agents cause nausea, vomiting, stomatitis, alopecia, and myelosuppression. Although most adverse effects are transient, certain ones (cardiac, pulmonary, and bladder toxicity) can be irreversible. Adverse effects can be minimized via supportive care therapy such as antiemetics for nausea and vomiting, erythropoietic agents and hematopoietic colony-stimulating factors for anemia and neutropenia, antihistamines and corticosteroids for hypersensitivity reactions, and chemoprotective agents such as mesna and amifostine for organ toxicity. A more intense measure involves harvesting bone marrow from a patient before myelosuppressive therapy and then reimplanting it after treatment.
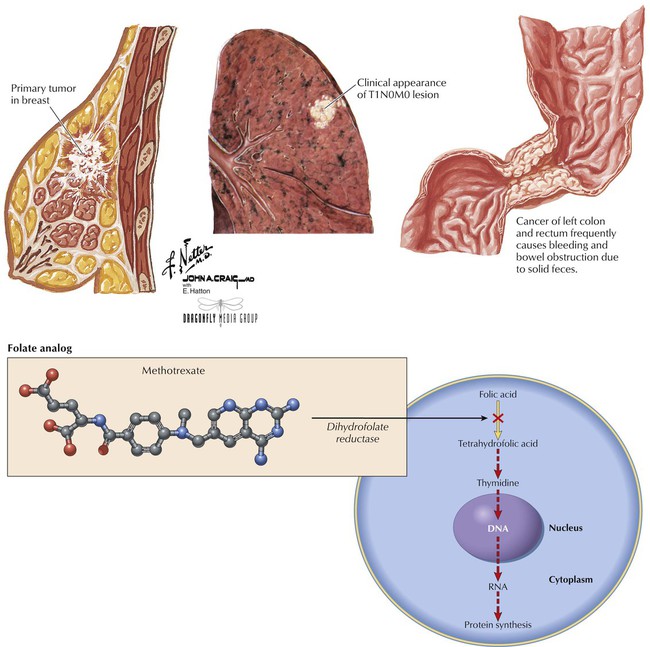
One of the oldest and most studied antineoplastic drugs, methotrexate (MTX) is structurally related to folic acid and is its antagonist: it inhibits dihydrofolate reductase (converts folic acid to the active tetrahydrofolic acid). Cells’ inability to use folate leads to reduced synthesis of thymidine and other building blocks (eg, DNA, RNA, proteins) essential to cell function. Cell death results. MTX is used for different cancers—eg, colorectal carcinoma, hematologic cancers (leukemias, lymphomas), and breast, lung, head, neck, and ovarian cancers. Common toxicities depend on dose: myelosuppression, erythema, stomatitis, alopecia, nausea, vomiting, diarrhea. More serious effects are hepatotoxicity, renal failure, and neurologic toxicity. Folic acid has no effect on MTX toxicity; leucovorin bypasses MTX-blocked dihydrofolate reductase, replenishes folate stores, and can prevent life-threatening neutropenia and mucositis, but it cannot protect against MTX-induced organ damage.
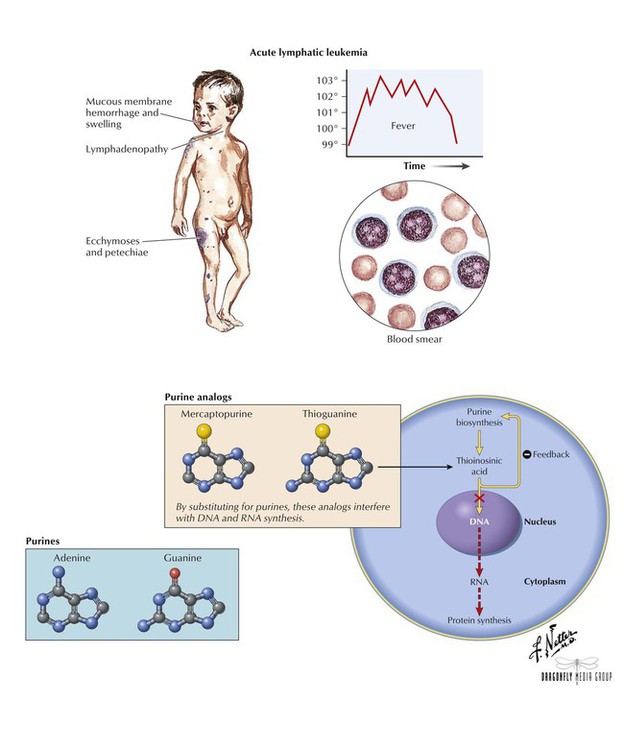
An analog of hypoxanthine and guanine, mercaptopurine (6-MP) is a prodrug that is converted in cells to active nucleotide metabolites. Thioinosinic acid is one such metabolite, which interferes with metabolic reactions needed for RNA and DNA biosynthesis. This metabolite also causes inhibition of the first step in purine biosynthesis or converts to another ribonucleotide that can cause feedback inhibition. 6-MP is primarily used to treat acute lymphatic (lymphocytic, lymphoblastic) leukemia. Adverse effects include dose-related bone marrow suppression, diarrhea, hyperpigmentation, hyperuricemia, and hepatotoxicity (when used with doxorubicin and at certain doses). The toxicity of oral 6-MP is increased when given with allopurinol. Thioguanine (6-TG) is also a purine analogue that is structurally and functionally related to 6-MP. Both agents share similar uses and toxicities. Unlike 6-MP, however, 6-TG is not potentiated by allopurinol.
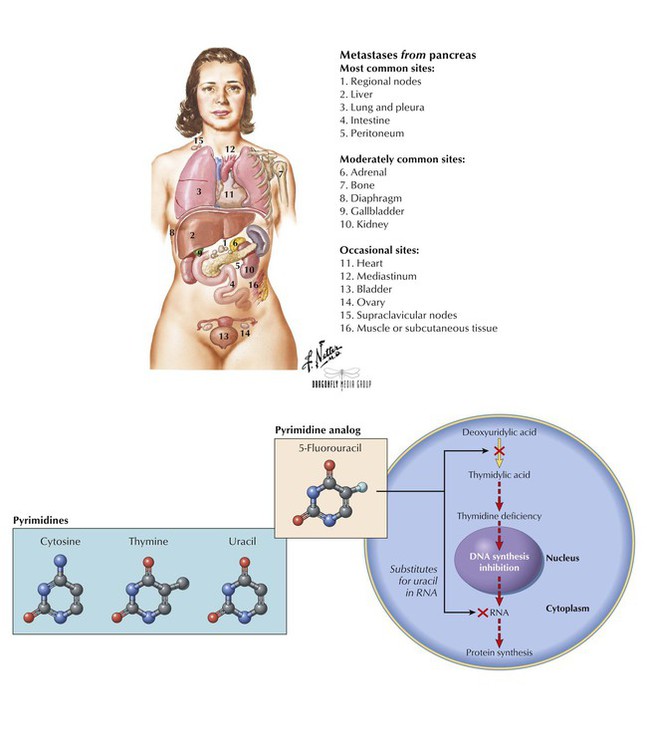
5-Fluorouracil (5-FU) is an inactive prodrug that, when converted to its active metabolite, inhibits methylation of deoxyuridylic acid to thymidylic acid, which leads to a lack of thymidine, a nucleoside of DNA. 5-FU also inhibits RNA formation by incorporating itself into the nucleic acid chain. The agent is used to treat solid tumors of the colon, rectum, breast, stomach, and pancreas. 5-FU is poorly absorbed orally and can cause severe GI toxicity, so it is given intravenously, intrahepatically, or topically. Blood dyscrasias, especially leukopenia, are the most common adverse effects; others are stomatitis and diarrhea, which can be severe in certain patients; hand-foot syndrome (painful, erythematous, swollen palms and soles); and cardiac toxicities (chest pain and tightness, dyspnea, cardiogenic shock). Alopecia is uncommon, and nausea and vomiting are usually mild.
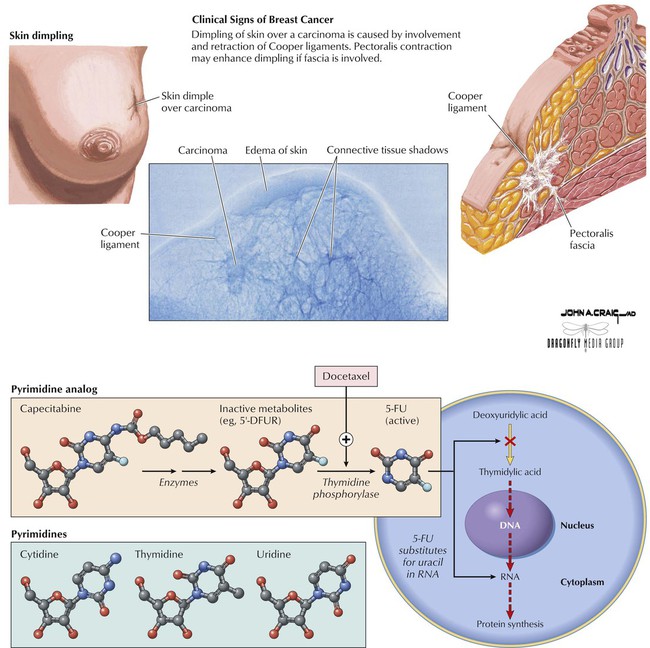
An oral, tumor-activated antineoplastic, capecitabine is a fluoropyrimidine carbamate that undergoes enzymatic conversion to inactive intermediates. When it reaches the tumor, it is converted to active 5-FU by thymidine phosphorylase, an enzyme that is found at high levels in tumors and low levels in normal tissues. This drug, together with docetaxel, is used for patients with metastatic breast cancer and failure to respond to previous anthracycline-containing therapy. It is also indicated as first-line therapy for metastatic colorectal carcinoma. This drug has selective tumor activation, so common drug-related adverse effects (eg, alopecia, bone marrow suppression) are minimized. Its most common side effects include diarrhea, nausea, vomiting, fatigue, stomatitis, and hand-foot syndrome. Potentially serious risks associated with the drug include severe diarrhea, grade 3 or 4 neutropenia, thrombocytopenia, and reduced hemoglobin levels.![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


