Drugs Used in Disorders of the Endocrine System
Overview
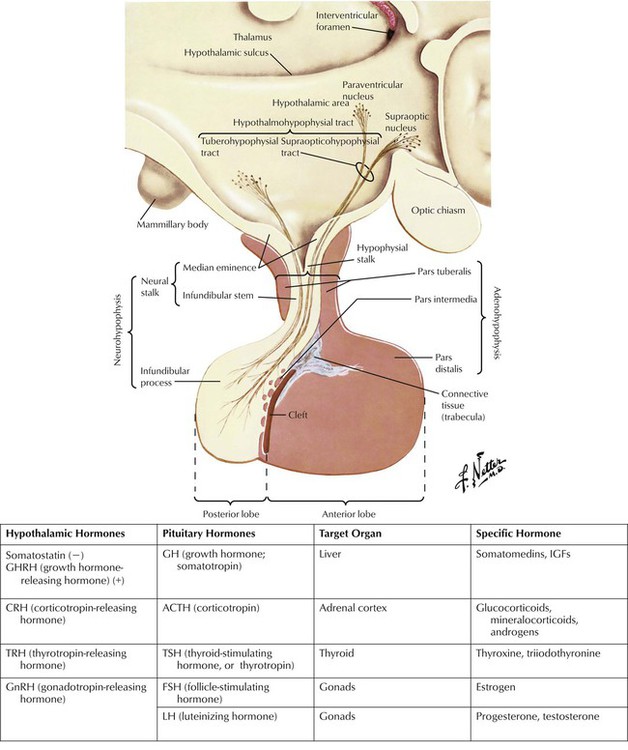
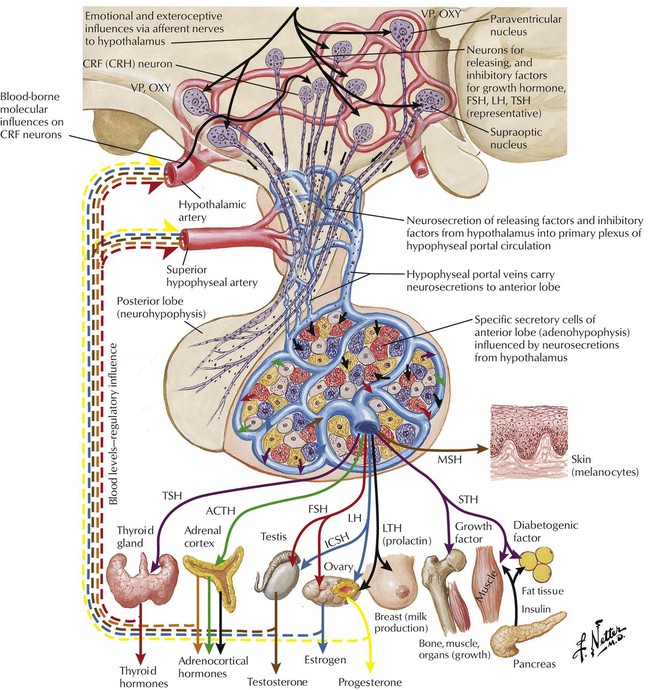
The hypothalamus and pituitary control a complex neuroendocrine system that governs metabolism, growth, and reproduction. The hypothalamus produces both inhibitory and releasing neuropeptides and hormones, which reach the pituitary via a hypophysial portal system. Hypothalamic hormones trigger release of anterior pituitary hormones, which are sent to target organs where they induce hormone synthesis. Most of these endocrine-organ systems function via negative feedback, eg, hypothalamic CRH stimulates pituitary ACTH secretion, which stimulates adrenal cortisol secretion, which in turn inhibits CRH and ACTH secretion. Hypothalamic and pituitary hormones are used as tools in stimulation tests to diagnose hypofunctioning or hyperfunctioning endocrine states. For example, ACTH and CRH, which target the adrenal cortex, aid adrenal insufficiency diagnosis. Pituitary hormones are also used as replacement therapy for deficiencies such as hypopituitarism.
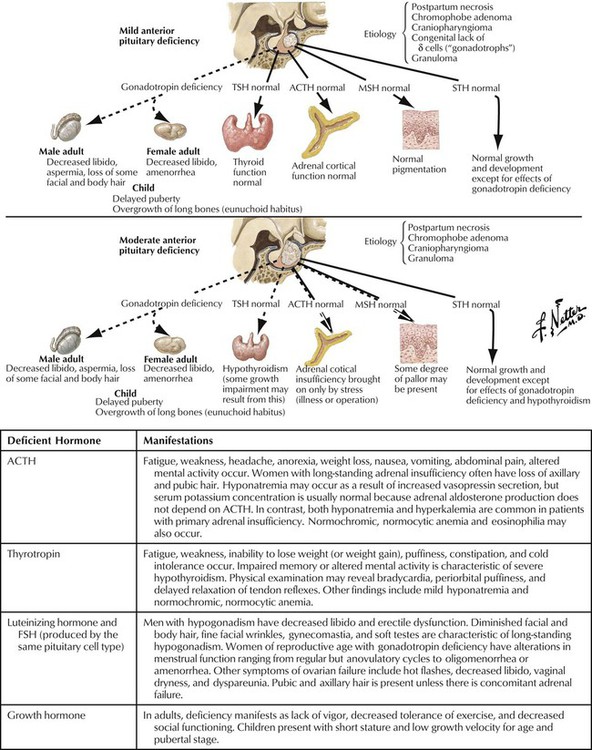
Hypopituitarism may be partial or complete and may result from hypothalamic disease (leading to deficiency of hypothalamic-releasing hormones) or intrinsic pituitary disease (causing pituitary hormone deficiency). Patients may present with, for example, adrenal insufficiency or hypothyroidism. Clinical signs depend on the degree and rapidity of onset of the deficiency. For example, basal cortisol secretion is normal in partial ACTH deficiency, but during an illness, adrenal insufficiency may occur. In complete ACTH deficiency, cortisol secretion is always subnormal. Diagnosis of complete deficiency is relatively easy: most patients have symptoms, and serum levels of target-organ hormone (eg, cortisol, thyroxine, and testosterone in men) and pituitary hormone (eg, ACTH, thyrotropin, and luteinizing hormone, respectively) are low. Causes of hypopituitarism include pituitary tumor (most common); hypothalamic tumor or cyst; infiltrative, vascular, and other disorders; and pituitary or cranial radiotherapy.
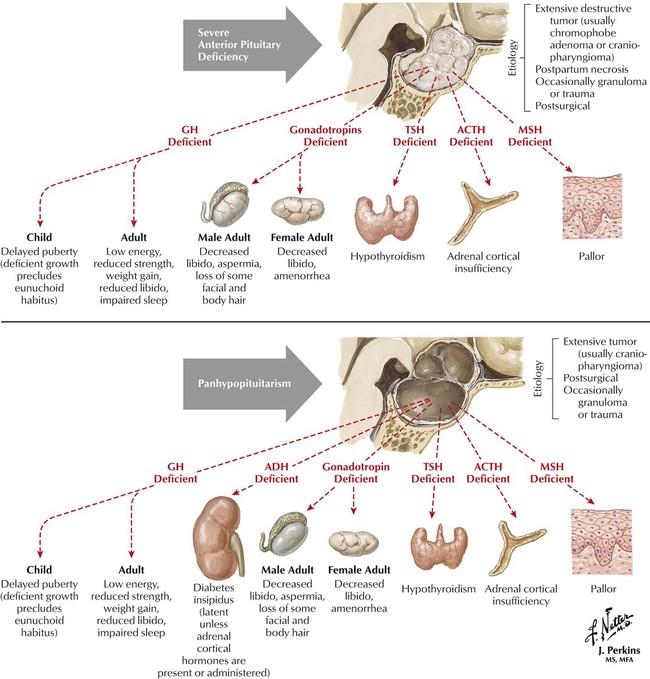
Growth hormone promotes linear growth by regulating endocrine and paracrine production of IGF-1. Besides disruption in growth, GH deficiency also causes increased subcutaneous visceral fat and reduced muscle mass, bone density, and exercise performance. Children have short stature and low growth velocity for age and pubertal stage. Adults, who usually have had pituitary tumors or head trauma, show low energy, reduced strength, weight gain, anxiety, reduced libido, and impaired sleep. GH therapy goals differ in children and adults. In adults, they are to improve conditioning and strength, restore normal body composition, and improve quality of life. In children, therapy promotes linear growth and restores body composition. Synthetic GH is effective for children with GH deficiency as long as epiphyses are not closed. Side effects include edema, muscle and joint pain, benign intracranial hypertension, hair loss, hypothyroidism, hypoglycemia or hyperglycemia, and the more serious risk of cancer.
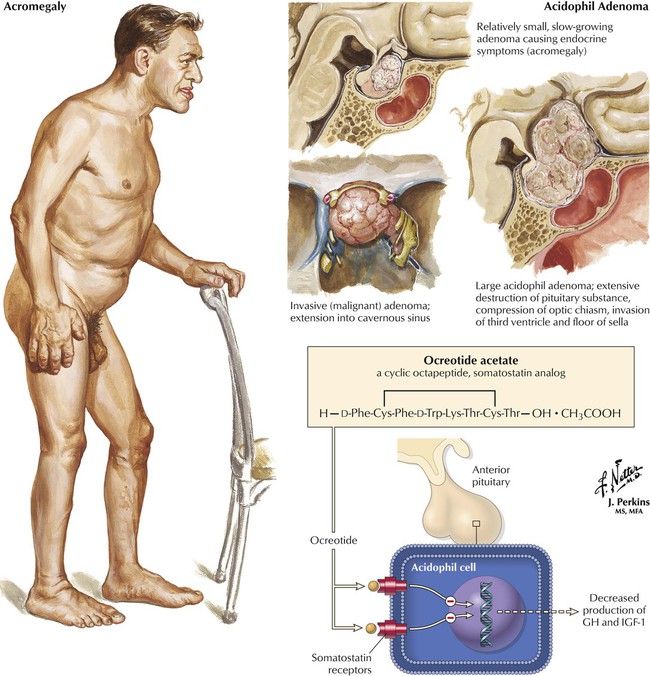
Acromegaly is a disfiguring hormonal disorder caused by excessive GH secretion from a pituitary tumor. Signs of acromegaly include coarse facial features and enlarged hands, feet, tongue, and internal organs (which lead to heart disease, hypertension, diabetes, arthralgias). Therapy includes surgical removal of the tumor and/or radiation, or subcutaneous use of octreotide, a GH inhibitor, available in a long-acting depot form. Octreotide effects mimic those of the natural hormone somatostatin (inhibition of GH and IGF-1 levels; suppression of the response of luteinizing hormone to gonadotropin-releasing hormone). By normalizing levels of GH and IGF-1—both markers for acromegaly—octreotide controls clinical signs and symptoms. Common adverse effects are gastrointestinal; the more serious effects include cardiac arrhythmias, hypoglycemia or hyperglycemia, suppression of thyrotropin, pancreatitis, and biliary tract abnormalities.
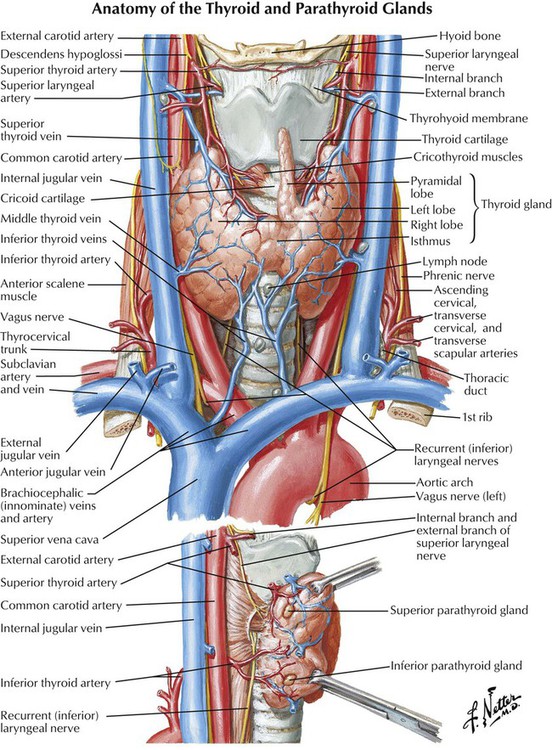
The thyroid gland is responsible for regulating normal growth and development by maintaining a level of metabolism in body tissues that is optimal for normal function. The thyroid synthesizes, stores, and releases 2 major, metabolically active hormones: triiodothyronine (T3) and thyroxine (T4). T3, the active form of the thyroid hormone, is 4 times more potent than T4, but its serum concentration is lower. Approximately 80% of the gland’s total daily production of T3 results from conversion of T4 to T3 through deiodination of T4. T3 and T4 exist in either free (active) or protein-bound (inactive) forms. More than 99% of circulating T4 is bound to plasma proteins, so only a small fraction exists in free form. As a result, T4 is metabolized very slowly and has a long half-life (7 days). T3 is less bound to plasma proteins and thus undergoes faster metabolism and has a shorter half-life (1.5 days).
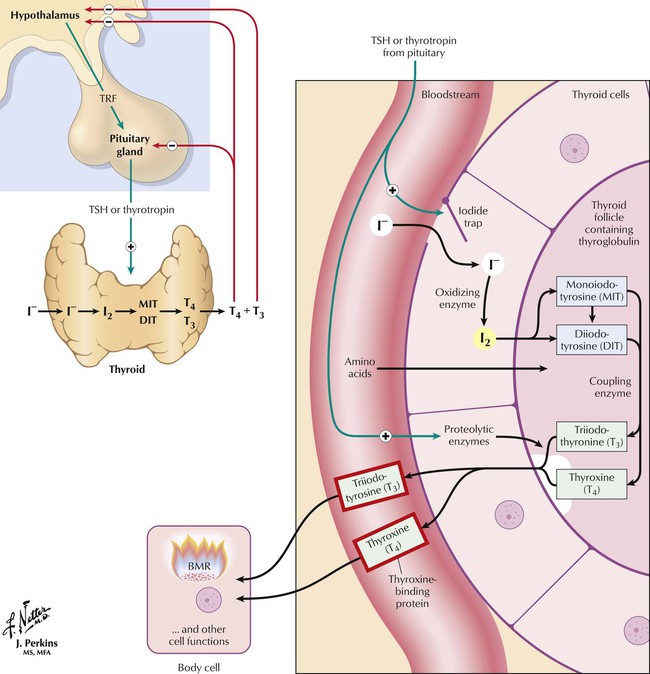
Thyroid hormones are synthesized and stored as amino acid residues of thyroglobulin. Major steps in synthesis and release include thyroid uptake of iodide, oxidation of iodide and iodination of tyrosyl groups of thyroglobulin, coupling iodotyrosine residues to produce iodothyronines, proteolysis of thyroglobulin, release of T4 and T3 into blood, and conversion of T4 to T3 in peripheral tissues and the thyroid. Hormone synthesis and release are controlled by a negative feedback mechanism (thyroid; hypothalamic-pituitary axis; autoregulation of iodide uptake). Low circulating hormone levels trigger hypothalamic release of thyrotropin-releasing factor (TRF), which induces pituitary secretion of thyrotropin (thyroid-stimulating hormone, TSH). Increasing TSH levels stimulate thyroid iodide uptake and hormone synthesis. Circulating hormones halt TRF and TSH secretion. The thyroid also regulates its own iodine uptake to protect against excess hormone production if extra iodide is ingested.
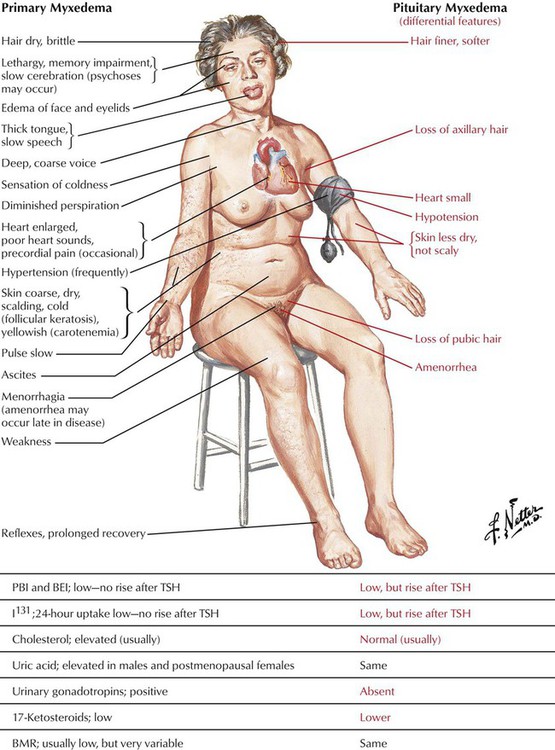
Hypothyroidism, a syndrome that results from a deficiency of thyroid hormones, can be caused by either primary (thyroid gland) or secondary (hypothalamic pituitary) dysfunction. The most common cause of primary hypothyroidism is Hashimoto thyroiditis, an autoimmune disorder in which unsuppressed T lymphocytes produce excessive amounts of antibodies that destroy thyroid cells. Certain drugs, such as lithium, nitroprusside, iodides, and sulfonylureas, can also induce hypothyroidism. The condition is usually more prevalent in females and persons older than 60 years. It typically presents with symptoms of “slowing down” (eg, weight gain, fatigue, sluggishness, cold intolerance, constipation, muscle aches). Goiter may be present. Patients with end-stage hypothyroidism or myxedema coma may experience hypothermia, confusion, stupor or coma, carbon dioxide retention, hyponatremia, and ileus. Laboratory findings include increased TSH and low free T4 levels.
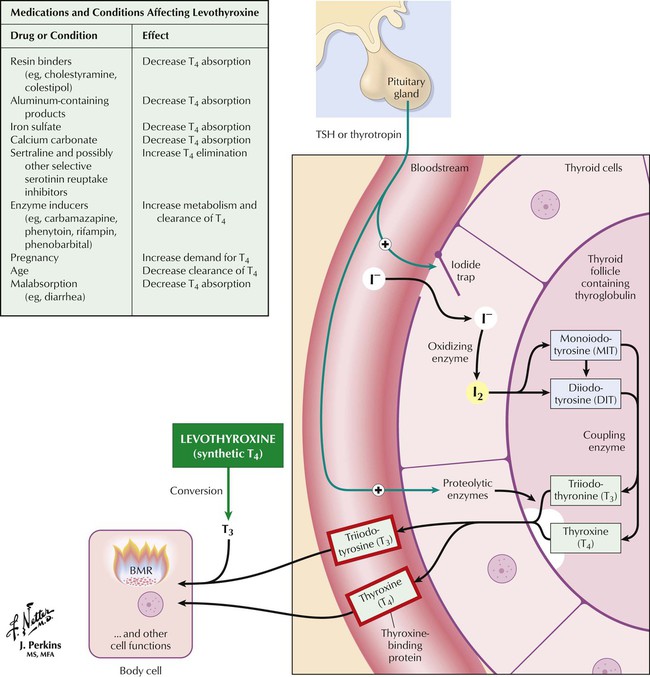
The principal treatment goal for hypothyroidism is to achieve a euthyroid state with thyroid replacement therapy. The preparation of choice is levothyroxine, a synthetic T4 formulation with advantages including stability, uniform potency, relatively low cost, once-daily dosing, and lack of foreign proteins. Levothyroxine may have innate metabolic activity, but most of its activity is due to its conversion to T3. Patients should notice improvement in typical symptoms of hypothyroidism after 3 to 4 weeks of treatment. Toxicity is directly related to T4 levels and manifests as nervousness, tachycardia, heat intolerance, and weight loss. Levothyroxine is available in various brands and generics, which may not be bioequivalent, so only 1 product should be used throughout treatment.
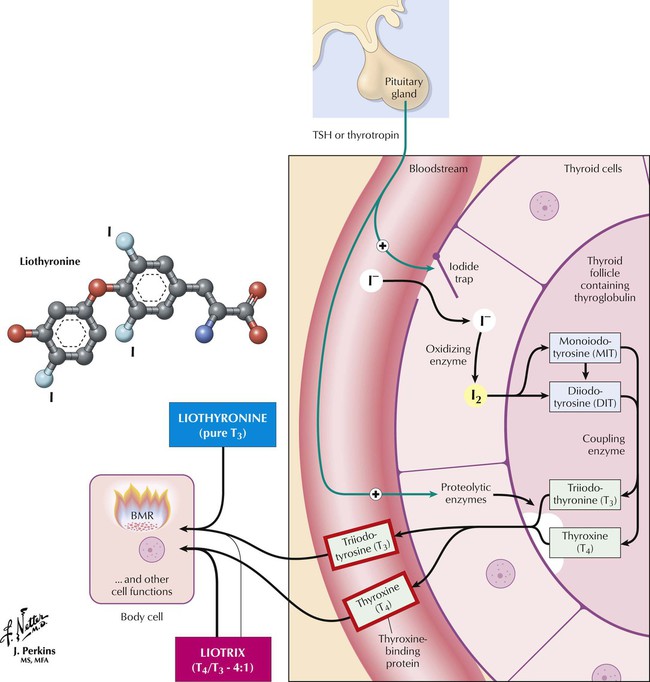
Liothyronine is a pure T3 preparation that is not recommended for routine thyroid replacement. After oral ingestion, T3 is absorbed more rapidly than T4, which may produce supraphysiologic plasma T3 levels, which can lead to thyrotoxicosis. Also, free T4 levels remain low during T3 administration and, if misinterpreted, could lead to incorrect use of more hormone. Therefore, T3 levels must be monitored. Other disadvantages are the need for multiple doses, higher expense, and greater potential for cardiotoxicity. T3 is therefore not better than T4, which is converted to T3 anyway. However, T3 is recommended for acute severe myxedema. Liotrix (a stable synthetic) and desiccated thyroid contain T4 plus T3. Liotrix uses a physiologic ratio of 4 : 1 but has the same problems as T3 and is more expensive. Desiccated thyroid, derived mostly from pork, is not recommended: product potency and composition vary and can result in toxic effects, including allergic reactions to animal protein.
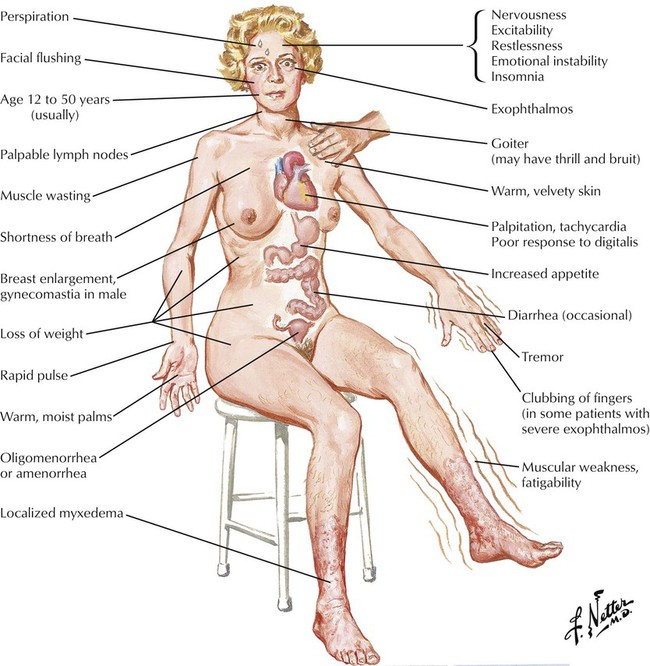
Hyperthyroidism, or thyrotoxicosis, is due to excessive thyroid hormone production and is characterized by increased metabolism in all body tissues. The most common cause of hyperthyroidism is Graves disease, an autoimmune disorder in which an abnormal thyroid receptor binds to the TSH receptor and causes uncontrolled thyroid hormone production. Drugs such as amiodarone, iodides, and lithium can also cause hyperthyroidism. Like hypothyroidism, hyperthyroidism occurs more often in females than in males. Symptoms include goiter, exophthalmos, nervousness, heat intolerance, palpitations, weight loss, insomnia, and new or worsening cardiac findings (atrial fibrillation, angina). Untreated hyperthyroidism can progress to thyroid storm, a possibly fatal state with acute onset of high fever, exaggerated thyrotoxicosis symptoms, cardiovascular collapse, and shock. Laboratory findings include high serum levels of free T4, undetectable TSH levels, or both.![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


