7 Drugs used in chronic conditions

Introduction
A large number of chronic diseases are brought about by demographic, environmental and lifestyle changes. Common conditions include asthma, diabetes, hypertension, Parkinson’s disease and epilepsy. This chapter will focus on the drug management of each of these conditions. The chapter is only introductory and is not intended as comprehensive coverage of all chronic conditions and their pharmaceutical management.
Asthma
Asthma is the leading chronic disease in developed countries and its incidence is increasing. The condition is brought on by a reversible obstruction of the airways which in turn causes the individual to become breathless and start to cough or wheeze. Asthma may be described as being acute or chronic. Severe acute asthma (known as status asthmaticus) is not reversed very easily and, as a result, continues to be fatal and requires prompt treatment. Chronic asthma usually has an intermittent pattern of breathlessness (dyspnoea), coughing and wheezing.
In acute asthma the main reason for obstruction of the airways is bronchoconstriction caused by a substance called histamine. Further significant obstruction of the airways is brought about by inflammatory changes in the mucosa which cause an increase in mucous production, more epithelial cells being shed and swelling of the tissues within the respiratory passages.
Asthma symptoms are thought to be caused by what doctors describe as hyper-reactivity or hyper-responsiveness of the cells lining the air passages. It seems that bronchiolar tissue in certain individuals is more sensitive to certain trigger factors than other people in the general population. Trigger factors include irritant chemicals, exposure to foreign proteins called allergens, and cold air.
Numerous cells and chemicals play a part in the changes that take place in the tissues of a person who suffers from asthma. We will now consider these changes in order to provide an understanding of how drugs used in this condition work.
Pathological changes in asthma
The changes in the respiratory system that bring about asthma are initiated by an allergic reaction. The trigger factor stimulates the body’s immune response and as part of this the body produces a type of white cell called a B-lymphocyte. Some of these B-lymphocytes mature into a type of cell called a plasma cell. These cells produce a chemical called immunoglobulin E which is a type of antibody. These antibodies in turn attach themselves to a type of tissue cell called a mast cell.
These mast cells are now stimulated to produce a substance called histamine. Histamine is a potent constrictor of the muscles in the air passages, causing them to become narrowed. This is called bronchoconstriction. Histamine also causes local blood vessels to dilate and become more permeable so that other inflammatory cells in the plasma can move into the tissues. Not only does histamine cause constriction of bronchial smooth muscle, it also leads to swelling of the tissues.
Another specific group of white cells thought to be involved in asthma are the eosinophils. This group of cells are implicated in the inflammatory response by releasing a range of chemicals that contribute to the contraction of bronchial smooth muscle, permeability of small blood vessels and excessive secretion of mucous. It is also suggested that the eosinophils produce chemicals that have a toxic effect on the epithelial lining of the airways and are responsible for the greater amount of epithelial shedding associated with this condition.
Macrophages are a type of bacteria-engulfing cell or phagocyte which are protective in function. However, in asthma these cells are responsible for releasing a group of substances called prostaglandins and platelet-activating factors which sustain the bronchial hyperactivity. This is thought to occur by increasing mucosal oedema, facilitating the number of eosinophils in the airway tissues.
The swelling of the local tissues from oedema is compounded by extra mucous production. Mucous is part of our general defence strategy in that it provides us with a mechanism to trap particles and bacteria and clear them from the upper respiratory tract. However, in asthma the size of the bronchial glands and goblet cells is increased and they subsequently produce large amounts of mucous which is thickened by cell debris from the epithelial shedding. The cilia that normally waft debris towards the pharynx also have their capability curtailed, contributing to the overall obstruction of the person’s air passages.
Asthma in children
Asthma is the leading cause of chronic illness in children. It affects as many as 15 per cent of children in the UK and, for unknown reasons, is steadily increasing. It can begin at any age, but most children have their first symptoms by age 5. Children still die from asthma − there were 27 recorded deaths in 2005 − and there is still significant disability associated with the disease, particularly severe childhood asthma, despite pharmacological advances.
There are many risk factors for developing childhood asthma, including:
- presence of allergies;
- family history of asthma and/or allergies;
- frequent respiratory infections;
- low birth weight;
- exposure to tobacco smoke before and/or after birth;
- being male;
- being black;
- obesity;
- being raised in a low-income environment.
No one really knows why more and more children are developing asthma. Some experts suggest that children are being exposed to more and more allergens such as dust, air pollution and second-hand smoke. These factors are all triggers of asthma. Others suspect that children are not exposed to enough childhood illnesses to build up their immune system, meaning that the body fails to make enough protective antibodies. Still others suggest that decreasing rates of breastfeeding have prevented important substances of the immune system from being passed on to babies.
Diagnosis of asthma in children is difficult because of the complex nature of the disorder in the young. In an adult a wheeze is a very important sign in the diagnosis of asthma, however in children there are many different causes of wheeze. In addition, children younger than five are generally unable to perform pulmonary function tests with the result that doctors rely heavily on history, symptoms and examination in making a diagnosis.

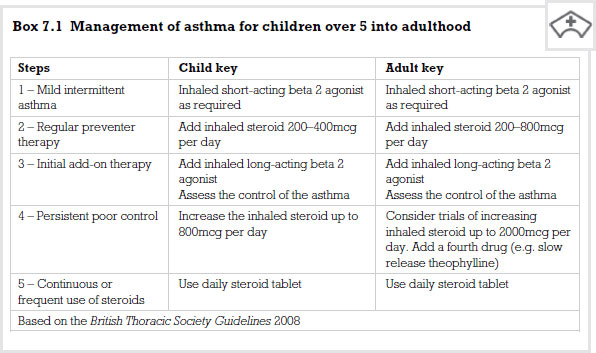
Drugs used in treating asthma
There are two basic drug groups given to patients who have asthma: bronchodilators and anti-inflammatory agents. They are not mutually exclusive – i.e. some bronchodilators may have an effect on inflammatory cells. Bronchodilators are the first group of drugs that we will focus upon.
Beta 2 adrenoreceptor agonists
These drugs are referred to as bronchodilators. They open the air passages by directly activating beta receptors, relaxing the smooth muscle in the walls of the bronchioles. This allows a more normal movement of air between the atmosphere and the alveoli. This group of drugs is available in both short- and long-acting forms. An example of a short-acting form in this class is salbutamol.

Most short-acting beta 2 agonists are available as aerosols to inhale by mouth several times a day. Most are also available as solutions that can be used with a special apparatus called a nebulizer. The maximum effect occurs within 30 minutes and the duration of action is between four and six hours. The patient is usually prescribed the drug on a ‘prn’ (pro re nata – as required) basis.

These medications are also available as dry powders, administered by inhalation. However, they are not prescribed on a prn basis but are taken regularly, such as twice a day. Sometimes you may find this type of drug being used alongside a glucocorticoid preparation.
Beta 2 adrenoreceptor agonists do not control the inflammatory response in asthma and if a patient is finding it difficult to control their symptoms by using this class of drug a corticosteroid preparation may be added. The most common side-effect of beta 2 adrenoreceptor agonists is tremor.
The drug from this family most commonly employed in the clinical setting is theophylline, which works as a bronchodilator by relaxing the bronchial smooth muscle. Several mechanisms of action have been proposed but it is still unclear how this drug produces its effects. Recently theophylline has also been shown to have some anti-inflammatory activity, especially with regard to mast cell activity. Theophylline is a useful drug in that it produces bronchial dilation so reducing the symptoms of chronic asthma, reducing the dosage of oral corticosteroids and reducing the requirement for symptomatic use of beta 2 adrenoreceptor agonists.
The drug is well absorbed from the GI tract and therefore can be given orally. Peak levels are achieved within one to two hours. However, if given with food, this will take longer. The drug has a relatively short half life and therefore you will come across a variety of sustained release preparations.
The drug is metabolized in the liver by a group of enzymes called cytochrome P450. Therefore, if the patient is taking other drugs using the same enzyme system, theophylline will accumulate. Two examples of drugs that might cause this interaction are erythromycin and ciprofloxacin.
Theophylline is used both in chronic asthma and chronic obstructive pulmonary disease (COPD, see p. 121) and as an emergency treatment in acute severe asthma. Despite its effectiveness it is not used as a first-line treatment but rather a third- or fourth-line option. One of the factors that limits its use is the high incidence of side-effects within its therapeutic range and narrow therapeutic index. As the plasma levels of the drug increase, so do the side-effects and concordance with this drug can be poor because of these side-effects. Theophylline has a stimulant effect on the central nervous system causing increased alertness, and can interfere with sleep. The drug also stimulates the cardiovascular system: the heart rate increases as does the force of contraction and thereby the patient’s blood pressure. Indigestion is also a common side-effect, probably due to the drug increasing gastric secretion and relaxation of the cardiac sphincter, therefore leading to reflux.
Deaths associated with theophylline have been reported and are due to the serious cardiovascular side-effect of dysrhythmia. Seizures are also a further life-threatening side-effect and can occur in concentrations only just above the therapeutic level.
Muscarinic receptor antagonists
The parasympathetic nervous system causes bronchoconstriction; therefore, if these effects on the bronchioles of the lungs were blocked dilatation would take place. This is the basic premise of this group of drugs. The compounds are used mainly in asthma, chronic bronchitis and COPD.
Ipratropium
This medicine comes as a solution to inhale by mouth, either as an aerosol or in a nebulizer. The aerosol is usually prescribed four times a day. The bronchodilator effect begins after approximately 45 minutes and lasts for about three to five hours. This drug can cause side-effects ranging from leaving an unpleasant taste in the mouth and/or a dry mouth to rashes, hoarseness and chest pain.

Inhaled corticosteroids
Glucocorticoids are dealt with in more detail in Chapter 5. They do not bring about bronchodilatation and are not given as an immediate response to the trigger factor. However, inhaled corticosteroids are now a regular therapy in asthma management. Two drugs commonly used are beclometasone dipropionate and budesonide. Both have anti-inflammatory and immunosuppressive activity. When inhaled they prevent the production of a chemical called arachidonic acid which in turn leads to the reduced formation of prostaglandins and leucotrienes, reducing the inflammatory response (see Chapter 5). Both drugs when taken regularly reduce the frequency of ‘rescue’ bronchodilator intake.
Inhaled corticosteroids are now preferred to oral steroids because of the reduced incidence of side-effects. However, side-effects do still occur but are usually only local in nature, for example sore and dry throat, and occasionally patients may develop oral thrush (candidiasis).


Cromoglicate
This medicine is not a bronchodilator, however if given prior to an asthmatic attack it prevents the immediate and later bronchoconstrictive reactions to inhaled allergens by stopping the release of mediators such as histamine from mast cells. It is thought that this medicine blocks calcium channels from opening in the mast cells. As calcium is blocked from entering the cells, so in turn is the release of inflammatory mediators. However, its exact mode of action is still not known.
Cromoglicate is given by inhaler, initially two puffs four times daily. For protection against bronchospasm caused by exercise the medicine should be used 15 to 30 minutes beforehand. If adequate response to the medicine is obtained then the dose may be reduced to two to six puffs daily.
The most frequently reported adverse reactions are throat irritation or dryness, a bad taste in the mouth, coughing, wheezing and nausea. Severe anaphylaxis and bronchospasm can occur but are fortunately rare.
Chronic obstructive pulmonary disease
COPD is a general term which includes the conditions chronic bronchitis and emphysema. COPD is the preferred term, but you may also hear it called ‘chronic obstructive airways disease’ (COAD). Chronic bronchitis and emphysema commonly occur alongside each other, leading to the airways becoming narrowed and a limitation of the flow of air to and from the lungs, causing shortness of breath. In clinical practice, COPD is defined by its characteristically low airflow in lung function tests. In contrast to asthma, this limitation is poorly reversible and usually gets progressively worse over time. In England, approximately 1 person in 59 is diagnosed with COPD at some point in their lives.
The most common cause of COPD is smoking. Once you give up smoking, you gradually reduce the chances of suffering from COPD, and you slow down its progress if you already have it. Occupational factors, such as coal dust and some inherited problems can also cause COPD. Whether pollution is a factor is under investigation.
A productive cough is usually the first symptom to develop, meaning that the patient produces sputum (phlegm). This cough tends to come and go at first, and then gradually becomes more persistent (chronic). The patient may actually refer to this cough as being a ‘smoker’s cough’ in the early stages of the disease. When breathlessness begins, people often become more concerned.
Breathlessness and a wheeze may at first occur only when the person is exerting themselves, such as when they climb the stairs. The symptoms become gradually worse over the years especially if the person continue to smoke. Difficulty with breathing may eventually become quite distressing.
Chest infections are more common in a person who has COPD. A sudden worsening of symptoms (such as when the patient has an infection) is called an exacerbation. Wheezing with a cough and breathlessness become worse than usual and the patient may cough up more sputum than normal. Such infections can be caused by bacteria or viruses. Bacteria (which can be killed using antibiotics) cause about one in two or three exacerbations of COPD. Viruses (not killed with antibiotics) such as the common cold virus may also be responsible for up to one in three exacerbations.
Stopping smoking is the most important treatment and no other may be needed if the disease is at an early stage and the symptoms are mild. If symptoms become troublesome, one or more of the following treatments may be advised (treatments do not cure COPD but they can ease the symptoms). As a general rule, a trial of one to three months of a treatment will give an indication as to whether it is helpful or not. The main treatments are medications in powdered form given via inhalers.
Diabetes
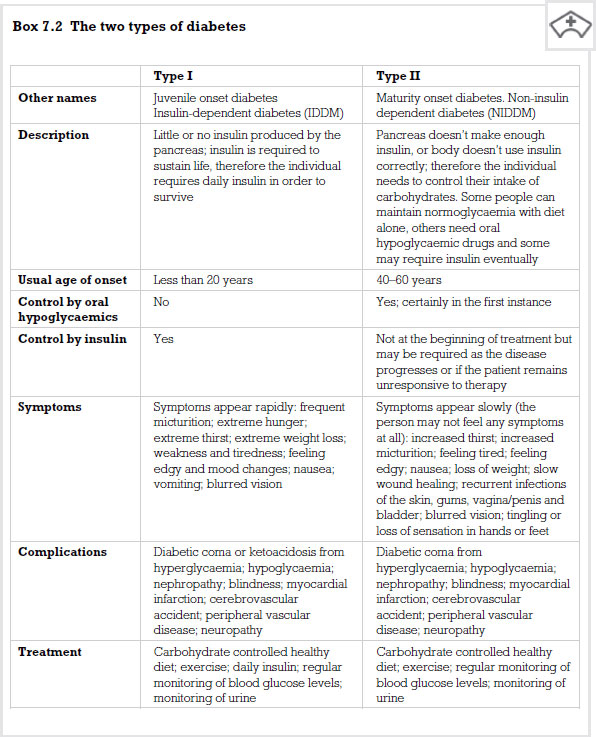
Diabetes mellitus is a chronic disease of metabolism caused by either an insufficient production of insulin in the body or an insufficient effect of insulin in the peripheral tissues. The main problems stem from a wide fluctuation in the range of blood glucose levels which leads to a number of short- and long-term difficulties for the individual. There are two main forms of diabetes: type I and type II.
In type I diabetes the body produces little or no insulin in cells contained in the pancreas and known as the ‘Islets of Langerhans’ or the beta cells. The pancreas is a specialized gland that lies just behind the stomach. Its functions range from regulating the amount of sugar (glucose) you have in your blood to producing enzymes that help break down and digest proteins, carbohydrates and fats. It is thought that the beta cells in the pancreas are destroyed by the body’s own immune response: hence diabetes is an autoimmune disease. Someone with type I diabetes will be required to take insulin for the rest of their lives.

In type II diabetes the beta cells may produce small amounts of insulin but not enough to control the levels of glucose in the plasma. In addition, the cells of the body, and particularly in the periphery, cannot utilize insulin properly. This means that glucose is not allowed to enter peripheral cells, so increasing the glucose levels in the plasma. This type of diabetes is linked to the problem of obesity.
It is estimated that approximately 2 million people in the UK are currently being treated for type II diabetes. However, researchers suggest that there are as many people who are under-diagnosed. The risk of this type of diabetes is also higher if it runs in the family or if the patient is overweight. From this perspective, it is clear that prevention of obesity will also lead to prevention of type II diabetes.
Without treatment, the main symptoms of diabetes can be divided into two major areas: acute symptoms and chronic complications. There are a number of symptoms that could be referred to as ‘classic’. These are excessive thirst (polydypsia), producing lots of urine necessitating many trips to the toilet (polyuria), blurring of vision, tiredness and weight loss due to muscle wasting (loss of muscle mass).
The chronic complications of diabetes are caused by long-term raised glucose levels in the body. This leads to damage, particularly to blood vessels and peripheral nerves.

Medicine management of diabetes
The overall aim of the treatment of diabetes is to maintain blood glucose levels within the normal range (normoglycaemia). This will relieve the acute symptoms and should minimize the impact of chronic complications on the individual. In reality, achieving normoglycaemia is very difficult and drug treatments are aimed at an individual level. An increase in exercise and cessation of smoking are advisable in order to reduce high blood pressure (hypertension) and high fat content in the blood (hyperlipidaemia). These measures have been shown to reduce the risk of long-term complications of this disease. However, the most important key to treatment of diabetes is diet. This can be used alone or can be combined with the use of insulin or other oral hypoglycaemic agents.
Insulin
The hormone insulin is released in the body as a direct response to a raised blood glucose level. Insulin is released directly into the blood and stimulates the uptake of glucose; it is the key that allows glucose to enter cells, and without it the door remains locked and glucose builds up in the blood. Insulin also promotes conversion of glucose to a substance called glycogen, which allows the body to store glucose for periods when the person is not eating but still requires glucose for bodily functions.
Insulin also limits the amount of fat breakdown in the body, a process called lypolysis. Triglycerides (large fat molecules) which are normally stored in your fatty (adipose) tissue are broken down into their constituent fatty acids and glycerol. These are then transported to the liver where they are used to fuel certain metabolic pathways. As insulin is either missing or low in quantity in diabetes these metabolic pathways soon become saturated, resulting in the production by the body of acidic compounds such as ketone bodies and acetate.
Treatment with insulin
At one time insulin was extracted from the pancreas of cattle (bovine insulin) or pigs (porcine insulin). However, today it is almost entirely human. This is possible through a process called recombinant DNA technology, which involves inserting the DNA code for human insulin production into bacteria so that they then produce commercial amounts of insulin.
Insulin is destroyed in the GI system which is why you never see it given by mouth as a tablet, for example.
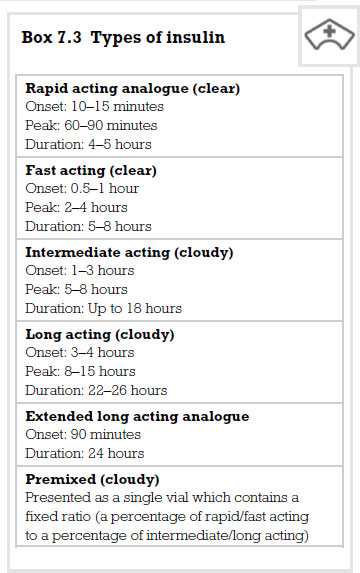
Once absorbed, insulin has a short half life of approximately 10 minutes. Therefore, for longer-term control, long-acting insulin is required (see p. 126).

Short-acting insulins
Soluble insulin produces a quick and short-lived effect. It works in the same way as natural insulin by binding to the receptors on the cells and allowing glucose to enter. It also causes the cells in the liver, muscles and fat to increase their uptake of glucose from the blood. Insulin lispro is a special form of human insulin which works more rapidly but for a shorter period of time. This enables people to inject themselves just prior to eating a meal if they so wish.
These types of insulins are the only ones suitable for IV injection.

Intermediate insulins
Longer-acting preparations of insulin are made possible by mixing the insulin with a substance which slows its use in the body. An example of this is isophane insulin which starts working about an hour and a half after administration. Its maximum effect occurs between four and eight hours after administration and ends as long as 24 hours afterwards.

Mixtard insulin contains a mixture of two types of insulin, soluble and isophane. Mixtard is available in a range of preparations containing these two insulins in differing proportions. Together, the combination is called biphasic isophane insulin. Premixed combinations provide a rapid initial lowering of blood glucose, followed by a more prolonged effect that lasts throughout the day. This is thought to mimic the body’s own insulin production more closely.
By mixing insulin with protamine and zinc an even longer action can be elicited. Lente and ultralente insulins start working after 6 hours and last for 24 hours. Insulin glargine is modified insulin and is opposite in its effect to insulin lispro in that it is designed to provide a constant insulin supply and mimic even more closely the body’s own physiological production of insulin. It is hoped that in conjunction with short-acting insulin a more normoglycaemic blood sugar can be maintained throughout a 24-hour period, lessening the risk of having a low blood sugar (hypoglycaemia) during the night. The National Institute for Health and Clinical Excellence (NICE) has endorsed the use of this insulin product in both type I and type II diabetes.
Side-effects
The main problem of giving insulin is that the blood sugar may be lowered too much, a state we call hypoglycaemia, which can cause brain damage. A person using insulin is three times more likely to be at risk from the effects of hypoglycaemia than a non-user.

Insulin pumps
An insulin pump is portable and attached to the patient. It consists of a main pump unit which holds an insulin reservoir (usually 3ml capacity like the cartridges used in an insulin pen). The reservoir is attached to a long, thin piece of tubing with a needle or cannula at one end which is known as the ‘infusion set’.
To use the pump, the cartridge is filled with fast-acting insulin and fitted inside. There is no need to take long-acting insulin because the insulin pump delivers constant amounts to the body. The needle or cannula is inserted under the skin and held in place with an adhesive patch, which fixes to the surrounding skin. The other end of the tube is connected to the pump which then delivers insulin through the infusion set according to its programming.
Using a pump, patients can instantly change the insulin dose, and fast-acting insulin is more easily absorbed by the body. Because the body gets a constant, regular flow of insulin, the effect is more constant also.
The bolus dose is designed to counteract the food being eaten. Therefore, when a diabetic eats a snack or at mealtimes the insulin pump can be programmed to provide an extra boost of insulin. Pumps can also be programmed to release a bolus dose over a longer period, which is be ideal for meals in restaurants and other similar occasions.
Oral hypoglycaemic agents
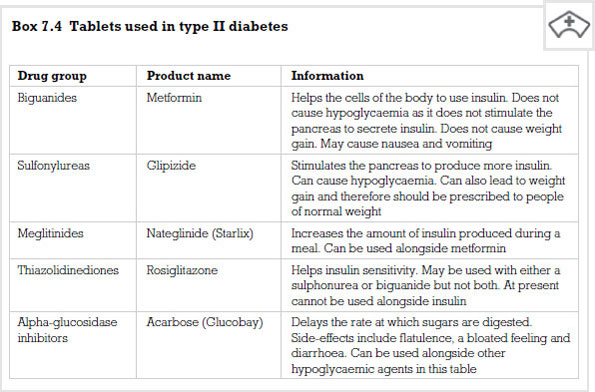
These are drugs that are usually given for type II diabetes. Dietary control certainly works for some people, however many others have difficulty in changing their lifestyles, especially on a permanent basis. For those people whose blood sugar is not controlled by diet alone there are a range of medicines available.
Metformin
Metformin is an antidiabetic medicine from a drug group called biguanides. It works in people with type II diabetes in three ways. First, it reduces the amount of glucose produced by cells in the liver. Second, it increases the sensitivity of muscle cells to insulin. Third, it delays movement of glucose from the GI tract to the blood following a meal. The cumulative effect of these processes is to lower the person’s blood glucose level.
Metformin is described as being a first-line medicine in the treatment of type II diabetes. It can be used in combination with other antidiabetic medicines to provide better normoglycaemia.
The drug has a half life of approximately three hours and a dose of 500mg three times a day with or after food is usually prescribed. The most common side-effects are anorexia (lack of appetite), diarrhoea and nausea. An increase in lactic acid in the blood is a rare but potentially fatal consequence of taking the medication. This is more common in patients who already have kidney, liver or heart failure who should not be prescribed the drug.
Sulfonylureas
This classification of drug is also used in the treatment of type II diabetes, particularly in people whose diabetes cannot be controlled by diet alone. They work by stimulating the beta cells in the pancreas to secrete insulin. There are two generations of this type of medicine, first generation drugs such as tolbutamide and second generation drugs such as glipizide.
Tolbutamide comes as a tablet and is usually taken twice or three times a day. The drug may lead to hypoglycaemia, but, of the first generation drugs, it is probably the safest, especially with the elderly. Its duration of action is approximately six hours and therefore is less likely to cause hypoclycaemia.
Glipizide is a more potent drug than tolbutamide, however both are equally effective in their overall hypoglycaemic ability. This drug is usually well tolerated by patients, but hypoglycaemia is a usual side-effect.

Oral hypoglycaemic agents such as tolbutamide and glipizide have been associated with increased cardiovascular mortality. However, there is no conclusive evidence that suggests that taking these drugs increases the risk of death from myocardial infarction.
There are some significant drug interactions with sulfonylureas, and certain drugs potentiate the hypoglycaemic effects. NSAIDs will lead to hypoglycaemia when given with sulfonureas. However, drugs such as thiazide diuretics and corticosteroids lessen the hypoglycaemic effects. This means that patients should have their blood glucose monitored more closely to ensure adequate control is maintained.
Meglitinides
Some drugs such as meglitnides that are not based on sulfonylureas have also been developed to expand the range of therapy. One such drug is nateglinide, which works in a similar way to sulfonylureas in that it enhances secretion of insulin from the beta cells in the pancreas. It is not as potent as most of the sulfonureas, is rapidly absorbed and has a fairly short elimination half life of three hours. This means that it has a short duration of action and there is therefore a lower risk of hypoglycaemia as a side-effect.

Thiazolidinediones (glitazones)
This class of drug came from a chance finding when it was being used as a fat-lowering treatment and it was noticed that thiazolidinediones also lowered blood glucose. The action of this class of drugs is complex. They reduce the breakdown of stored glucose in the liver and increase the amount of glucose entering muscle tissue. Insulin that is circulating is enhanced by these drugs. The accumulation of these actions leads to a lowering of the blood glucose level. They do not cause hypoglycaemia as a side-effect when used on their own but may do so if combined with insulin or a sulfonurea.
It is believed that this class of drugs also lowers fats that are thought to be implicated in the build up of fatty plaques in blood vessels (atherosclerosis). The only tablet in this group is pioglitazone. Its cardiovascular safety profile compares favorably with rosiglitazone (Avandia), which was withdrawn after concerns about an increased risk of cardiac events. Pioglitazone is used on its own or alongside metformin and/or another antidiabetic medicine to treat type II (non-insulin dependent) diabetes mellitus. It can also be used with insulin. Pioglitazone comes as a tablet to take by mouth. It is usually taken once daily with or without meals. It should be taken at around the same time every day.

Common side-effects include nausea, difficulty in focusing, ankle swelling, increase in appetite and weight gain. Pioglitazone and other similar medications for diabetes may cause or worsen congestive heart failure (a condition in which the heart is unable to pump enough blood to the other parts of the body).
Alpha-glucosidase inhibitors
A drug called acarbose is used from this family of medicines to treat type II diabetes. It works by slowing down the action of chemicals in the body that break down foods which release glucose. Slowing food digestion helps to keep the blood glucose from rising very high after a meal.
The medicine is given as a tablet, usually three times a day. It is important that the patient takes each dose with the first bite of their meal. Side-effects include flatulence, diarrhoea, abdominal pain and bloating due to the amount of unabsorbed carbohydrates left in the GI system. Acarbose is not a treatment for diabetes that you will encounter often in a hospital setting although it is an option for patients.
Hypertension
Hypertension (high blood pressure) is associated with a risk of coronary heart disease. Increased pressure in the blood vessels has a direct effect on the heart and vasculature. One of the major problems of having hypertension is that it has few symptoms and individuals often only discover they have the condition on routine screening. The effects of raised blood pressure have a pronounced effect on the heart, particularly the left ventricle. This increases in size (ventricular hypertrophy) as it attempts to pump against an increased peripheral resistance.
The arteries also respond to the increased pressure within them by enlargement of their muscular walls. This hypertrophy both in the heart and vessels leads to cardiac failure as a muscle cannot restore its normal contractility.
The risk of cerebral vascular accident (stroke) also rises significantly with elevation in systolic and diastolic pressures. Hypertensive disease is also associated with the formation of fatty plaques in the walls of blood vessels, a condition we call atherosclerosis. This is a Catch 22 situation as atherosclerosis leads to further hypertension. The combination of high pressure and artherosclerosis leads to weakness in the walls of arteries and increases the risk of them rupturing. The blood vessels most commonly affected are the cerebral, coronary and renal vessels. Therefore, as well as the increased risk of cerebral vascular accident and myocardial infarction, the individual is also likely to suffer from renal disease.
Hypertension may be defined as primary or secondary. In secondary hypertension the elevated blood pressure is caused by renal or endocrine disease. However, the majority of cases of hypertension are primary or ‘essential’ and this condition is still not fully understood. The causation of hypertension is most likely to be due to a number of interrelated factors which may be genetic or environmental.
Regardless of cause, hypertension is a fatal, silent killer, unless treated. In most instances the disease process extends over a long period of time and this is known as benign hypertension. However, in a small minority of cases the disease progresses very rapidly and is very difficult to control. This is referred to as malignant hypertension and is usually triggered by kidney disease arising from essential hypertension or other causes.
Arterial pressure is regulated by the performance of the heart (measured as cardiac output) and the diameter of the arterioles (total peripheral resistance). Hypertension involves an increase in either cardiac output or the peripheral resistance. Mechanisms that seem to be involved affect the extracellular fluid volume and expand the circulating blood volume. This involves excessive renin secretion from the kidney. Renin is a hormone that raises blood pressure in the body. Increased sympathetic activity which activates stress hormones and an excessive dietary salt intake, possibly associated with a low potassium intake, are also implicated in the pathogenesis of this disease.
Non-pharmacological remedies for hypertension are usually the first to be prescribed before commencing medicines. Weight reduction, salt restriction, moderation in alcohol consumption and cessation of smoking are all recommended before prescribing treatment. A GP may also prescribe a regime of moderate exercise which has been shown to be beneficial in helping to lower systolic pressure. Relaxation and biofeedback are also techniques that are advisable prior to drug therapy. It is important that the disease is diagnosed and treated early as intervention, be it pharmacological or non-pharmacological, can reduce the risk of much cardiovascular disease associated with hypertension.
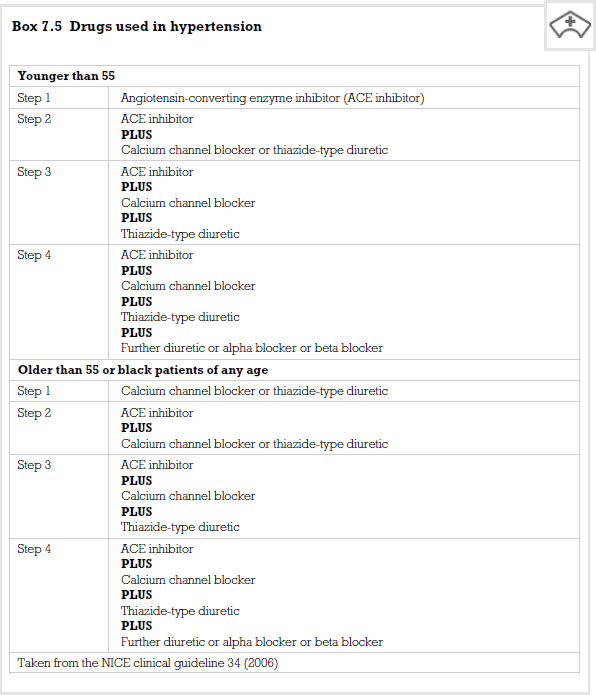
In the past, treatment of hypertension involved giving patients medicines that had many side-effects, however, as medicine has progressed so has the pharmacology. Generally, patients are commenced on a thiazide diuretic. If this does not give adequate control then the patient may be prescribed drugs from the beta-adrenoreceptor antagonist (beta blocker) group. If the blood pressure remains elevated then the individual would progress onto more potent groups of drugs such as angiotensin-converting enzyme inhibitors, or calcium antagonists (calcium channel blockers). Finally a blockade of alpha adrenoreceptors would be commenced. Typically, drug regimes for people with hypertension consist of a number of these drugs used in combination.
Thiazide diuretics
These are usually the first-line treatment for mild to moderate hypertension, providing there are no contraindications. A drug that is used commonly is bendroflumethiazide. You may come across other diuretic drugs such as frusimide. This works on a different part of the kidney (loop of henle). It is a more powerful diuretic and is not usually used for the treatment of hypertension.
Bendroflumethiazide works by stopping the body from selectively reabsorbing sodium. This has an effect on the circulating blood volume causing it to decrease. It has also been noted that this group of diuretics has an effect on blood vessels, causing them to vasodilate and lower blood pressure.
Bendroflumethiazide is well absorbed from the GI tract so is usually given as a tablet. A dose of 2.5mg daily is usually prescribed. Higher doses do not necessarily mean a greater fall in blood pressure, but would increase the side-effects. The maximum effect takes place at four to six hours and its duration of action is between 8 and 12 hours.
Problems can occur with this drug. First, it increases the amount of uric acid present in the plasma because it prevents that acid from being secreted from the body. Therefore, anybody suffering from gout should not take the medicine. Another common side-effect is potassium depletion.

Beta-adrenoreceptor antagonists
In practice these drugs are usually referred to as beta blockers. If a patient develops hypertension then taking this group of medicines will cause a gradual fall in arterial pressure. However, this will take several days or weeks to occur. How these drugs work is not fully understood but they appear to have actions that reduce the cardiac output, reduce renin production from the kidney and reduce the release of stress-related hormones such as adrenaline.
In the body you have at least two types of beta-adrenoreceptors, some in the heart (beta1 receptors) and some in the lungs (beta2 receptors). Beta blockers are either selective, whereby they stop the effect of adrenaline at beta1 receptors, or non-selective whereby the drug blocks both types. This is a consideration as beta2 receptors bring about bronchodilation. Therefore, blocking this effect in patients with airway disease is not desirable. Such patients should be given a selective drug such as atenolol.
This group of medicines can all be given in tablet form. Some are taken once daily (e.g. atenolol) and others need to be taken more often (e.g. propranolol). Serious side-effects are not common but are important when giving information to patients.
Patients with diabetes need to be informed that they might not recognize when they are going hypoglycaemic. The signs and symptoms of hypoglycaemia are mediated through the sympathetic nervous system. This group of drugs inhibits sympathetic responses and the patient may therefore get little warning of impending lowering of blood glucose levels.
Due to the fall in circulating blood volume, peripheral perfusion of tissues is a consideration. Often a person will complain of having cold hands and feet. Therefore, patients with peripheral vascular disease pose a particular problem and this type of medicine should be avoided. Other side-effects may include fatigue, a low pulse rate (bradycardia) and very vivid dreams and nightmares.

Angiotensin-converting enzyme inhibitors
These are known as ACE inhibitors and work by stopping the powerful vasoconstricting effects of angiotensin II on the blood vessels. In order to become active angiotensin II has to convert from angiotensin I. This group of drugs, which includes captopril, stops this conversion from taking place, therefore lowering the blood pressure.
It is important that the patient drinks plenty of fluids while on these drugs. Dehydration lowers the blood pressure further, so causing an unwanted severe hypotensive state. Captopril can be used on its own or in combination with other antihypertensive agents. It is a powerful hypotensive drug and therefore patients usually start on a low dose.
Sometimes it is advisable to take the initial dose of the drug before going to bed, so removing the chances of faints or falls due to hypotension.
A dry cough from irritation of the bronchial mucosa is a common side-effect with some patients going on to experience bronchospasm. Captopril has certain individual side-effects due to the nature of its biochemistry. It can cause rashes, changes in taste, a lowering of a group of white cells called neutrophils (neutropenia) and protein to appear in the urine (proteinurea).
Calcium antagonists
These drugs act by limiting the amount of calcium that enters smooth and cardiac muscle. This causes the blood vessels to dilate, therefore causing the blood pressure to fall. Some of these drugs act better on the heart muscle (e.g. verapamil) and others work better on smooth muscle of the blood vessels (e.g. nifedipine and amlodipine).
Calcium antagonists are well absorbed by the GI system and are given in tablet form. However, drugs differ in how short- or long-acting they are and this has an effect on dosage and unwanted side-effects. For example, nifedipine has a short half life and needs to be taken more frequently than amlodipine which is taken once a day. However, slow release preparations of nifedipine are available.
Unwanted side-effects are an extension of the drug’s therapeutic activities. Shorter-acting medicines may cause flushing and headaches and if used over a long period of time can cause ankle swelling. Apart from these predictable side-effects these drugs are relatively trouble-free.
Alpha-adrenoreceptor antagonists
A drug called prazosin was the first selective alpha antagonist to be developed and was used for many years. However, better drugs have now been developed, for example doxazosin and terazosin. These can be taken once a day.
Like beta blockers, these drugs work by inhibiting the action of adrenaline and noradrenaline on body systems. The drugs mentioned above are highly selective, which means they can affect vessels, so causing dilatation, but do not speed up the heart rate (tachycardia). Neither do they lead to any appreciable severe lowering of blood pressure when standing (postural hypotension). Perhaps for these reasons that they have become more popular, especially in combination with other antihypertensive therapies.
Parkinson’s disease
This is a progressive disorder of movement. Certain neurones in part of the brain called the basal nuclei degenerate and this leads to a lack of a neurotransmitter called dopamine. Typically, this disease occurs in people who are over the age of 50. The reduction in the release of dopamine causes the basal nuclei to become overactive and this overactivity presents itself in a number of ways. The patient develops a persistent tremor, even at rest, and quite often there are head-nodding and pill-rolling movements of the fingers. Their posture becomes affected and they begin to bend forward and walk in small, shuffling steps. Textbooks used to describe the person’s face as ‘mask-like’ because they acquire a stiff facial expression. Finally, the patient has trouble in initiating and coordinating movement.
Parkinson’s disease was first described by James Parkinson in 1817. In 1960 Hornykiewicz demonstrated that the dopamine content of the substantia nigra in post-mortem examination of patients with the disease was extremely low – in some cases 10 per cent less than normal. More sophisticated diagnostic tests have revealed similar findings and suggest the loss of dopamine occurs over several years. Symptoms only show themselves when the dopamine content of this area has fallen below between 20 and 40 per cent of the normal.
With this knowledge, one would expect to be able to find a cure. It would seem on the face of it that all we need to do is replace the lost dopamine and the patient would return to a normal state. Drugs that are given for this debilitating disease do work by increasing dopamine in the brain in a number of ways, however despite these pharmacological interventions patients do not return to a normal state. Many deteriorate and suffer dementia. In addition to the affects of the disease there are also side-effects to the medication.
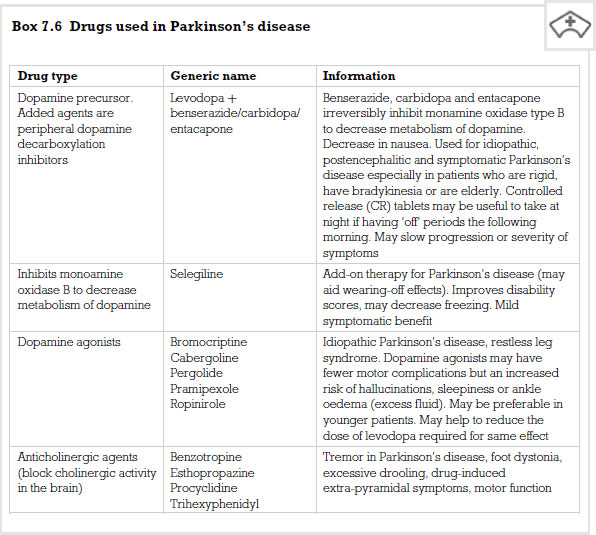
This remains the first line treatment for Parkinson’s disease. You cannot give pure dopamine as the BBB will not allow it to enter and it is also destroyed quickly by enzymes in the peripheral tissues. Levodopa is a drug that can cross the BBB and then be converted by the central nervous system into dopamine. It is nearly always combined with another substance that stops peripheral enzymes breaking down dopamine. This means that generally more levodopa is available to the brain and also a lower dose of dopamine can be given as most of it remains intact rather than being destroyed by enzymes. Examples of medicines that are combined or given with levodopa are carbidopa and benserazide.
When given levodopa the response rates of patients are good. Some studies suggest that 20 per cent of patients may actually be restored to normal function for a while. However, this improvement is often only short-lived and as time goes by the levodopa becomes less effective. This loss of efficacy is probably due to the unrelenting course of the disease.
Two major side-effects are associated with giving levodopa. First, a condition called dyskinesia can develop which means that the individual develops a series of involuntary movements, causing acute embarrassment as they usually affect the face and limbs. If the dose is lowered, the dyskinesia does stop, but is replaced by the rigidity it had improved. This is a fine line that the patient and doctor walk in order to accept the consequences of both illness and treatment.
A second side-effect associated with this drug group is called the ‘on-off ‘ effect. This is where, quite suddenly, the drug therapy seems to stop working. This can be quite distressing for the patient and can sometimes occur when they are in the middle of doing something. The reason for this fluctuation is not fully understood, however, patients should be made aware of both these side-effects when commencing treatment.
The patient may also suffer more short-term side-effects from the medication, though these quite often improve over a period of time. They may feel sick, have no appetite and suffer a slight drop in blood pressure. This decrease in blood pressure could have a more serious effect if the patient is having antihypertensive therapy as they could develop postural hypotension.
A small number of patients may develop delusions and hallucinations as the brain is given extra dopamine which is thought to mimic the high levels found in patients with schizophrenia.
Selegiline
This medicine works by stopping the enzyme that breaks down dopamine in the synapses, therefore making more dopamine available. The enzyme is called monoamine oxidase-B and appears only in regions of the brain which are dopamine rich. This means that the drug only works in these areas of the brain, so limiting any side-effects or interactions. Giving this drug with levodopa seems to have better results than giving levodopa on its own. However, when levodopa is given in combination with either carbidopa or benserazide, there is no difference noted between this and giving selegiline only.
Dopamine receptor agonists
This group of drugs works by mimicking the action of dopamine on dopamine receptors. Examples of this class are bromocriptine and pergolide. Their duration of action is longer than that of levodopa so they do not need to be given as often. The toxic effects of these drugs are similar to levodopa, however, the schizophrenic-type symptoms can be more severe. Sometimes these drugs are given in combination with levodopa in order to reduce overall side-effects or improve the patient’s response.
Acetylcholine antagonists
Prior to the discovery of levodopa this group of drugs was the main treatment for patients suffering from Parkinson’s disease. Dopamine works opposite to acetylcholine in the nigra striatel system, therefore if dopamine is lacking, by stopping the actions of acetylcholine, balance can be restored albeit at a lower level. This is the thinking behind acetylcholine antagonists. The action of drugs like benzatropine is more limited than levodopa in that they do not reduce rigidity significantly.
These drugs also have troublesome side-effects that can lead to glaucoma and urinary retention in an elderly person. As a result their use is usually confined to treating side-effects of drugs given as neuroleptic agents (e.g. chlorpromazine). However, with the advent of atypical antipsychotic medication their use is in decline. Refer to Chapter 8 for more on drugs that are used in mental health.
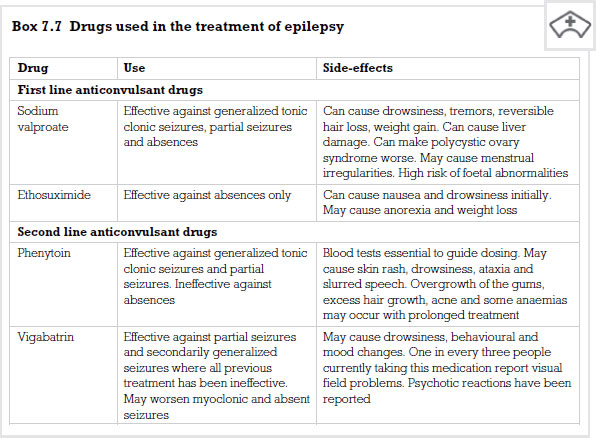
Epilepsy
Epilepsy is the most common chronic disabling condition of the nervous system. Epileptic seizures result from an imbalance of the excitatory and inhibitory mechanisms within the brain, the nerve impulses generated from the neurones being abnormal and uncoordinated. The form of seizure that the person suffers usually depends on the part of the brain affected. Seizures can range from brief lapses in attention to full-blown convulsive fits which may last up to several minutes. The classification of epilepsy recognizes two major categories: partial and generalized.
Partial seizures
These tend to occur in a localized area of the brain. The symptoms depend on which brain region is discharging these abnormal impulses. Simple partial seizures are an example of this, where the motor cortex is irritated, resulting in twitching. The patient does not lose consciousness. The twitching sometimes spreads from a small area of the body to a larger area as the impulse spreads across the motor cortex. This is called a Jacksonian seizure.
Complex partial seizures usually originate from the temporal lobe and are preceded by what is called an aura or warning. The person then commences to enter into a period of altered behaviour where they repeat movements over and over again.
Examples of this may be rubbing, patting or plucking activities. Patients may also describe alterations in sensation like a smell or a sense of being there before (déjà vu). These seizures may last a few minutes, after which the person has no memory of the events. The behaviours during these seizures can be bizarre and accompanied by strong emotional responses, for example aggression.
Generalized seizures
These involve the whole brain and affect the reticular formation centres responsible for consciousness and arousal. Abnormal impulses arise throughout both hemispheres. Two important categories are absent seizures which used to be called petit mal and tonic clonic seizures which used to be called grand mal.
Absent seizures are characterized by a brief alteration in consciousness which is sometimes very difficult to see. This type of seizure happens more in children and can be frequent in nature.

Tonic clonic seizures are vastly different. These consist of an initial strong contraction of the whole muscular system causing a rigid spasm. Respiration ceases with defecation, micturition and salivation often occurring. This tonic stage lasts for approximately a minute. The person then begins to suffer a series of violent rhythmic jerking movements of the limbs. They usually develop an increased heart rate (tachycardia) and begin to sweat. This phase lasts about two to four minutes, followed by a coma. The person is usually sleepy after the seizure and often cannot remember the event.

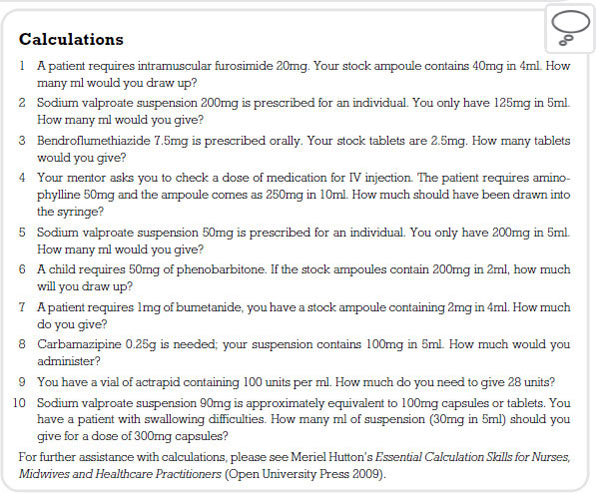
Treatment for epilepsy is aimed at producing a life free of seizures. In order for this to be achieved patients are prescribed a range of drugs which are called anticonvulsants. These medicines work in one of three ways. Some block sodium channels in the neurone, others block calcium channels and the third group enhance an inhibitory neurotransmitter called gamma-aminobutyric acid (GABA).
Inhibition of sodium channel function
In Chapter 3 we discussed the firing of a neurone (see p. 32) and the importance of the passage of sodium into the neurone. It can be deduced that if we block this action in some way, then neurones will be less likely to fire. Sodium-inhibiting drugs do not block all sodium channels but are selective to channels that allow high frequency impulses to take place. In other words, they dampen down the excitability in neurones without switching them off. An example of this class of drug is phenytoin.
Phenytoin
This drug is still widely used but not for absent seizures, which may get worse if the drug is administered. It is well absorbed by the body when given orally but has certain characteristic pharmacokinetic tendencies which must be taken into consideration before it is prescribed. First, it binds to plasma proteins in the blood, which is not unusual for drugs to do, however when giving drugs that unblock this mechanism (e.g. salicylates) there is an increase of the phenytoin in the person’s plasma. Phenytoin also increases enzymes into the liver that can affect the breakdown (metabolism) of other drugs that the patient may be taking. This process is called enzyme induction. For example, if the patient is taking oral anticoagulants the effect of these will be unpredictable if phenytoin is also given.
Phenytoin works to its full potential between 40 and 100 micromoles per litre. If the range exceeds this the patient gets more toxic side-effects without any further protection from seizures. This is why it is important to impress upon the patient the need for regular blood monitoring while on this therapy.
Side-effects begin to appear when the dose pushes the blood plasma levels to over 100 micromoles per litre and become severe if the blood plasma increased beyond 150 micromoles per litre. The patient may complain of feeling dizzy (vertigo), have headaches and their movements may become shaky. They may have difficulty walking (ataxia). In some cases people begin to have involuntary rapid movement of their eyes, a condition we call nystagmus. If the blood levels begin to rise further, the patient becomes confused and there is marked intellectual impairment. One particularly unpleasant side-effect of this drug is the development of swelling of the gums. This is called hyperplasia and can often be disfiguring and therefore acutely embarrassing.
Phenytoin has also been responsible for malformations in babies born to women taking the drug. Problems such as cleft palate were increased more than in the general population, therefore if a woman falls pregnant while taking the medication she should seek professional advice immediately.
Inhibition of calcium channels
Some of the anticonvulsant drugs are thought to have minor involvement in blocking calcium channels. Indeed, gabapentin, a fairly recent addition to the anticonvulsant range, may have some action on calcium channels. The only drug known for its action purely on calcium uptake in the neurone is ethosuximide.
Ethosuximide
This is the first line management drug when it comes to treatment for absent seizures. It has little or no effect in other types of epilepsy and can sometimes actually increase the risk of seizures.
The drug works on a certain class of calcium channels. It is thought that through their action they moderate the firing of neurones in an area of the brain called the thalamus. The abnormal firing of neurones in this area seems to play a role in the generation of absent seizures. The dose of the drug in adults is usually 0.5–2g daily. In children it falls to 10–20mg per kg once a day.
Side-effects are relatively minor but obviously troublesome if you are the one taking the medication. They include nausea, anorexia and sometimes lethargy and dizziness. Very rarely the drug can have severe hypersensitivity reactions.
Enhancement of GABA action
Sodium valproate
It is not entirely clear exactly how valproate works as it does several things in the brain. First, the chemical messenger (or neurotransmitter) GABA is ‘inhibitory’ on the brain – i.e. it calms the brain down. Once it has worked there are other chemicals (or enzymes) in the brain which are there to break GABA down so that it can no longer work. In people with normal levels of GABA this prevents there being too much GABA. In some people it is thought that there may not be enough GABA in the brain. This lack seems to ‘trigger’ fits or overactivity. Valproate helps to stop the breakdown of GABA and so leaves enough of this chemical in the brain to help prevent fits.
Second, it may inhibit ‘repetitive firing’ of neurones. When a message is passed, there is a short refractory period or gap (about one thousandth of a second) before the next message can be passed, during which time the nerve ending resets itself. Valproate may increase this refractory period or time by a small amount. Under normal circumstances, this will make no difference at all, but if the brain is overactive and lots of messages are being passed in quick succession the valproate will reduce the number of messages back to the normal level (e.g. if the next message follows along before the nerve has reset itself, the message can’t be passed).
Compared with other anticonvulsant drugs, valproate is relatively free of unwanted side-effects, however the patient should be informed that they may feel sleepy to start with and so need to take extra care when driving or operating any type of machinery. This effect should wear off after the patient has been taking the medicine for a while. Valproate can cause some people to become more hungry than usual and they may then put on weight. Some patients may put on weight even without eating more. The patient should be informed to see a dietician for advice if weight gain becomes problematic.

The most serious side-effect is poisoning of the liver (hepatotoxicity). For the first six months of treatment the patient will need a regular blood test (e.g. every month) to check that the drug is not affecting their liver. The patient may also need to have blood tests from time to time to make sure that the dose of valproate is enough and not too much or too little for them.
It is also important for the patient to consider that there will be a risk from taking this medicine during pregnancy. Major malformations occur spontaneously in about 2–4 per cent of all pregnancies, even if no drugs are taken. A medicine causing teratogenicity (foetal malformation) is called a teratogen. Since a baby has completed its main development between days 17 and 60 of the pregnancy (the so-called ‘first trimester’), these first 2 to 16 weeks are the main concern. After that, there may be other problems (e.g. some medicines may cause slower growth). The infant may also be affected after birth (e.g. withdrawal effects are possible with some drugs). There is some evidence of problems of this nature when taking valproate (e.g. a 1 in 100 chance of spina bifida and a ‘valproate syndrome’). Therefore this drug is not normally given in pregnancy.
Vigabatrin
Vigabatrin works by stopping the breakdown of GABA in the brain by the enzyme GABA transaminase. The drug is extremely specific for this enzyme, so enhancing the inhibitory effects of GABA in humans. It is well absorbed from the GI tract and is therefore given as a tablet. It has a short plasma half life but because it blocks the GABA-destroying enzyme irreversibly it can be given once daily. The body will obviously make more GABA transaminase so the drug has to be given continuously. The main side-effects are depression and psychotic disturbances.


Recommended further reading
Beckwith, S. and Franklin, P. (2007) Oxford Handbook of Nurse Prescribing. Oxford: Oxford University Press.
Bourke, S.J. (2007) Respiratory Medicine, 7th edn. Malden, MA: Blackwell.
Brenner, G.M. and Stevens, C.W. (2006) Pharmacology, 2nd edn. Philadelphia, PA: Saunders Elsevier.
Campbell, D.A. (2006) Respiratory nursing: understanding difficult-to-control asthma, Nursing Times, 102(36): 44–5.
Capaldi, B. (2005) Treatments and devices for future diabetes management, Nursing Times, 101(18): 30–2.
Clark, A. (2002) Spotlight: drug information factsheet – diuretics, Professional Nurse, 17(8): 484.
Clayton, B.D. (2009) Basic Pharmacology for Nurses, 15th edn. St. Louis, MO: Mosby Elsevier.
Coben, D. and Atere-Roberts, E. (2005) Calculations for Nursing and Healthcare, 2nd edn. Basingstoke: Palgrave Macmillan.
Cradock, S. (2004) Essential reading on type 1 diabetes, Nursing Standard, 18(49): 29.
Cradock, S. and Skinner, C. (2001) Managing type 2 diabetes: a dynamic approach, Nursing Times, 97(7): 42–3.
Doran, C. (2000) Learning curve: managing epilepsy, Nursing Times, 96(19): 37–8.
Downie, G., Mackenzie, J. and Williams, A. (2007) Pharmacology and Medicines Management for Nurses, 4th edn. Edinburgh: Churchill Livingstone.
Durham, S.R. (2008) ABC of Allergies. Malden, MA: Blackwell.
Everett, J. (2004) The role of insulin pumps in the management of diabetes, Nursing Times, 100(16): 48–9.
Gallimore, D. and Jordan, S. (2004) Prescription drugs, uses and effects: ACE inhibitors – cardiovascular disease, Nursing Standard, 18(45).
Gatford, J.D. and Phillips, N. (2006) Nursing Calculations, 7th edn. Edinburgh: Churchill Livingstone Elsevier.
Hainsworth, T. (2005) NT clinical: how to maintain optimal glycaemic control in diabetes, Nursing Times, 101(48): 19–20.
Hewitt, J. and Jordan, S. (2005) Antiepileptics: neurology, Nursing Standard, 19(49).
Hill, J. (2003) Diabetes: devices for insulin administration, Nursing Times, 99(15): 51–2.
Hill, J. (2005) The use of insulin to improve treatment in type 2 diabetes, Nursing Times, 101(28): 32–4.
Hillson, R. (2002) Practical Diabetes Care, 2nd edn. Oxford: Oxford University Press.
Jarvis, J., Burden, A. and Burden, M.L. (2004) An RCT into calculating insulin start dose in type 2 diabetes, Nursing Times, 100(21): 40–4.
Jordan, S. and Lake, R. (2005) Insulin, Nursing Standard, 19(32).
Karch, A.M. (2008) Focus on Nursing Pharmacology, 4th edn. Philadelphia, PA: Lippincott Williams & Wilkins.
Lapham, R. and Agar, H. (2003) Drug Calculations for Nurses: A Step-by-step Approach, 2nd edn. London: Arnold.
Levy, D. (2006) Practical Diabetes, 2nd edn. St. Albans: Altman Publishing.
Lowey, A. (2005) Drug treatment of type 2 diabetes in adults, Nursing Standard, 20(11): 55–64, 66, 68.
McLoughlin, C. (2004) Diuretic drugs, Professional Nurse, 20(2): 50–1.
Nair, M. (2007) Diabetes management: nursing management of the person with diabetes mellitus – part 2, British Journal of Nursing, 16(4): 232–5.
Newell, K. and Hume, S. (2006) Choosing the right inhaler for patients with asthma, Nursing Standard, 21: 46–8.
NICE (National Institute for Clinical Excellence) (2002) Clinical Guideline G: Type 2 Diabetes, Blood Glucose. London: NICE.
NICE (National Institute for Clinical Excellence) (2002) Clinical Guideline H: Type 2 Diabetes, Management of Blood Pressure and Blood Lipids. London: NICE.
NICE (National Institute for Clinical Excellence) (2003) NICE approval for epilepsy drugs, Nursing Times, 99(46): 6.
NICE (National Institute for Clinical Excellence) (2004) Clinical Guideline 5: Chronic Obstructive Pulmonary Disease. London: NICE.
NICE (National Institute for Clinical Excellence) (2004) Clinical Guideline 15: Type 1 Diabetes. London: NICE.
NICE (National Institute for Clinical Excellence) (2004) Clinical Guideline 20: Epilepsy. London: NICE.
NICE (National Institute for Health and Clinical Excellence) (2006) Clinical Guideline 34: Hypertension. London: NICE.
NICE (National Institute for Health and Clinical Excellence) (2006) Clinical Guideline 35: Parkinson’s Disease. London: NICE.
Noble, C. (2006) Modes of drug delivery used to manage Parkinson’s disease, Nursing Times, 102(32): 30–2.
Nursing Times (2003) What you need to know about … asthma, Nursing Times, 99(18): 28.
Nursing Times (2004) What you need to know about … insulin. Nursing Times, 100(8): 29.
O’Connor, B. (2001) Inhaler devices: compliance with steroid therapy, Nursing Standard, 15(48): 40–2.
Phillips, A. (2007) Experiences of patients with type 2 diabetes starting insulin therapy, Nursing Standard, 21(23): 35–41.
Phillips, A. (2007) Starting patients on insulin therapy: diabetes nurse specialist views, Nursing Standard, 21(30): 35–40.
Rees, J. and Kanabar, D. (2006) ABC of Asthma, 5th edn. Malden, MA: Blackwell.
Roberts, J. and Williams, A. (2004) Quality-of-life and asthma control with low-dose inhaled corticosteroids, British Journal of Nursing, 13(19): 1124–9.
Rodgers, J. (2000) Clinical pharmacological interventions in type 2 diabetes – the role of the nurse, British Journal of Nursing, 9(13): 866–70.
Rodgers, J. and Walker, R. (2002) NT plus: glycaemic control in type 2 diabetes, Nursing Times, 98(19): 56–7.
Scullion, J. (2005) A proactive approach to asthma, Nursing Standard, 20(9): 57–66, 68.
Shuttleworth, A. (2004) NT clinical: implementing new guidelines on epilepsy management, Nursing Times, 100(45): 28–9.
Simonson, T., Aarbakke, J., Kay, I., Coleman, I., Sinnott, P. and Lyssa, R. (2006) Illustrated Pharmacology for Nurses. London: Hodder Arnold.
Thomas, S. and MacMahon, D. (2002) Continuing professional development: Parkinson’s disease – managing Parkinson’s disease in long-term care, Nursing Older People, 14(9): 23–30.
Thomas, S. and MacMahon, D. (2004) Continuing professional development: Parkinson’s disease, palliative care and older people – part 1, Nursing Older People, 16(1): 22–6.
Thomas, S. and MacMahon, D. (2004) Continuing professional development: Parkinson’s disease, palliative care and older people – part 2, Nursing Older People, 16(2): 22–6.
Trounce, J. (2000) Clinical Pharmacology for Nurses, 16th edn. New York: Churchill Livingstone.
Wagstaff, S. (2004) Understanding the importance of effective glycaemic control, Nursing Times, 100(44): 45.
Wallymahmed, M. (2006) Insulin therapy in the management of type 1 and type 2 diabetes, Nursing Standard, 21: 50.
Watkins, P.J. (2003) ABC of Diabetes, 5th edn. London: BMJ Books.
Watkins, P.J., Amiel, S.A., Howell, S.L. and Turner, E. (2003) Diabetes and its Management, 6th edn. Malden, MA: Blackwell.
Wehrle, L. (2003) Epilepsy: its presentation and nursing management, Nursing Times, 99(20): 30–3.
Wolfe, S. (2006) Respiratory nursing: treatment of allergic rhinitis and asthma, Nursing Times, 102(20): 49–52.
< div class='tao-gold-member'>